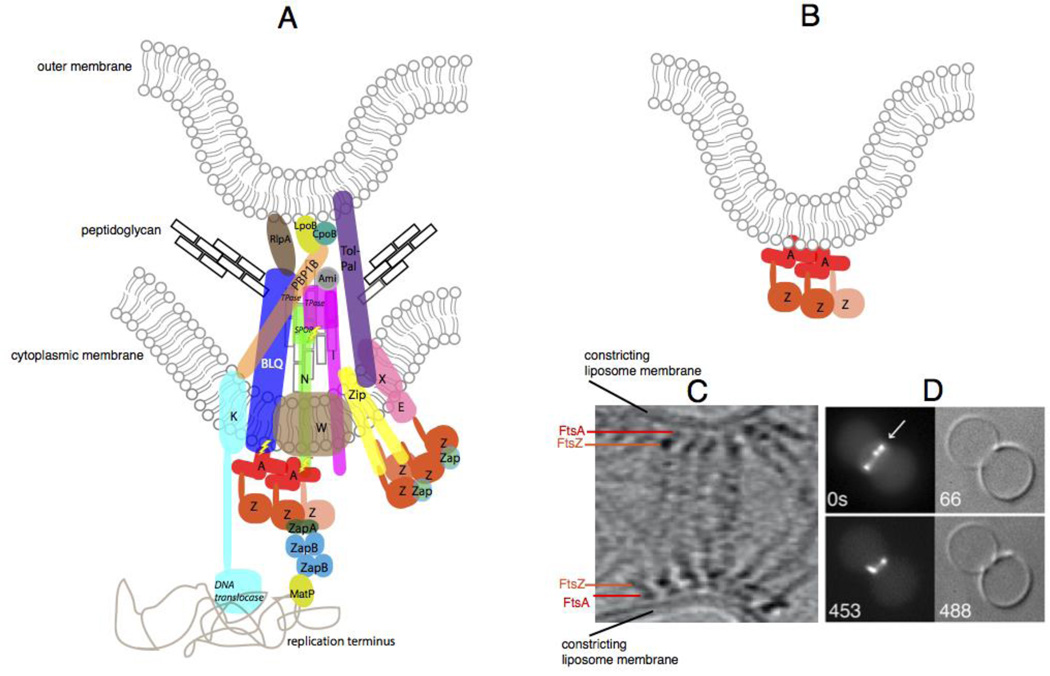
Fig. 4.
The constricting divisome in vivo and reconstituted in vitro. (A) A schematic of a putative individual sub-complex at the constricting divisome of E. coli shows FtsZ polymers being anchored to the membrane by FtsA and ZipA, along with other essential and nonessential divisome proteins of E. coli that cross the inner membrane, some of which contact the peptidoglycan and the outer membrane. Sidewall and septal peptidoglycan are denoted by black or grey rectangles, respectively. All Fts proteins are shown as a single letter, including the FtsBLQ complex. FtsZ dynamics are shown by the lighter colored FtsZ monomer coming off the polymer. Most known direct protein-protein contacts are shown, but some are speculative. Known enzymatic activities are highlighted in italics (TPase = transpeptidase). ZapC and ZapD are denoted as “Zap”, and the several periplasmic amidases of E. coli are denoted as “Ami”. Also shown are contacts made between the divisome and the terminus region of the nucleoid, including the helicase domain of FtsK155 and MatP, which probably interacts with ZapB 156. Regions of putative regulatory signaling are shown with lightning bolts. (B) Schematic of constriction of liposomes in vitro by FtsZ tethered to FtsA in the liposome lumen. (C) Cryo-ET of T. maritima FtsZ and FtsA proteins pinching a liposome from the inside reproduced with permission from Ref 47. and D) and fluorescence/DIC microscopy of E. coli FtsZ-YFP and FtsA* also pinching liposomes from the inside; reproduced with permission from ref 109.