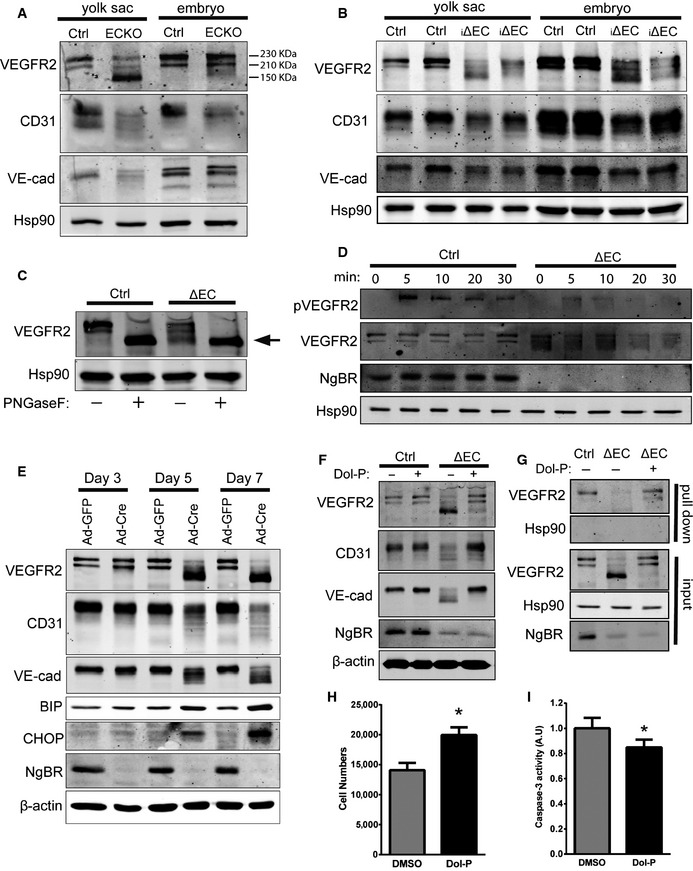
-
A
Western blot analysis of NgBRECKO embryos and yolk sac lysates to detect cell surface glycoproteins. Most VEGFR2 in the mutant yolk sac was detected at ˜150 kDa instead of at 230 or 210 kDa as in the control.
-
B
Western blot analysis of NgBR
i∆
EC embryos and yolk sac lysates to detect cell surface glycoproteins.
-
C
Analysis of N‐glycosidase F (PNGaseF)‐treated NgBR
∆
EC lysates. The arrow indicates non‐glycosylated VEGFR2. PNGaseF cleaves between the innermost GlcNAc and asparagine residues of high mannose, hybrid, and complex oligosaccharides from N‐linked glycoproteins.
-
D
VEGFR2 phosphorylation in response to VEGF. pVEGFR2 Y1175 was detected after stimulation with VEGF (50 ng/ml) by immunoblot analysis.
-
E
Western blot analysis of endothelial cell and ER stress marker proteins in primary MLEC. Isolated MLEC from NgBR
f/f were treated with Ad‐GFP or Ad‐Cre, and lysates were collected at day 3, 5, and 7 after virus infection.
-
F
Rescue of EC glycosylation defects with Dol‐P treatment. After 2 days of Ad‐GFP or Ad‐Cre infection of NgBR
f/f
MLEC, Dol‐P (50 μg/ml) was added for additional 72 h. Cell lysates were collected and EC protein glycosylation detected by Western blotting. Data are representative of 3–5 independent experiments.
-
G
Dol‐P rescues surface expression of VEGFR2. Surface protein biotinylation of MLEC infected with Ad‐GFP (Ctrl) incubated with DMSO and Ad‐Cre (i∆EC) incubated with DMSO or Dol‐P (50 μg/ml). HSP90, a control intracellular protein, was present only in the input fraction.
-
H, I
Proliferation (H) and caspase‐3 activity (I) of NgBR‐deleted MLEC with Dol‐P supplementation. *P < 0.005. P‐values were calculated by unpaired Student's t‐test. Data are mean ± SEM from 3 independent experiments.