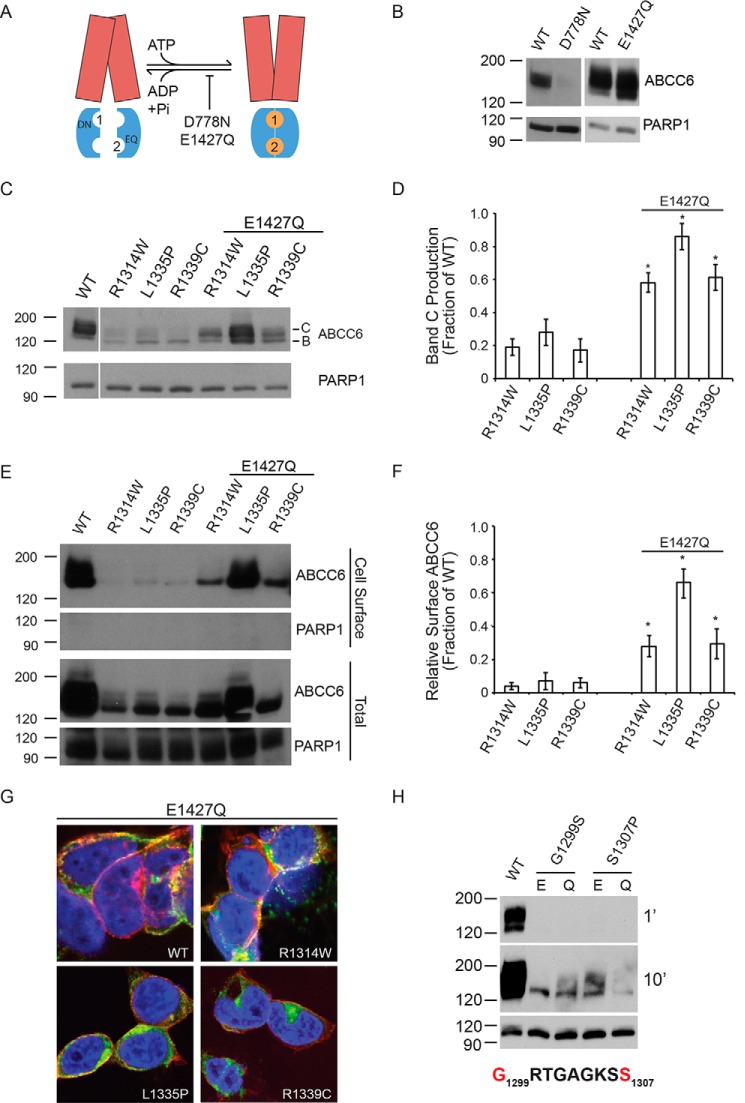
FIGURE 3.
Rescued ABCC6 folding by domain-domain stabilization. The catalytic E1427Q mutation was used to stabilize the ATP-bound NBD dimer and to assess the rescue of ABCC6 folding. A, a schematic showing the putative domain-domain stabilization by the E1427Q mutant is shown. The catalytic mutation, E1427Q, putatively blocks ATP hydrolysis at the NBD2 Walker A/B composite site. The TMDs are shown in red, the NBDs are shown in blue, and the ATP is shown as orange spheres. The ATP molecules are numbered based on their association with the NBD1 or NBD2 Walker A/B sequences. B, representative Western blots of the NBD1 and NBD2 catalytic mutants in the otherwise wild type ABCC6 background are shown. C, representative Western blots of the E1427Q double mutants are shown. D, densitometric analysis of Western blots of steady state ABCC6 is shown. E, representative Western blots of cell surface biotinylated ABCC6 are shown. F, densitometric analysis of Western blots of steady state ABCC6 is shown. G, confocal immunofluorescence images of the E1427Q mutants are shown. ABCC6 is shown in green, WGA is shown in red, and DAPI is shown in blue. Yellow indicates colocalization of the ABCC6 and agglutinin staining. H, representative Western blots of Walker A ATP-binding site mutations are shown with Glu-1427 (E) or E1427Q (Q). Western blots and immunofluorescence images are representative of n ≥ 4 independent experiments. The identities of band B and C are indicated in C. PARP1 is shown as a loading control in B, C, E, and H. Data shown are summary or representative of n ≥ 3 independent experiments. Quantified data are mean ± S.D. *, p < 0.01 using ANOVA with Tukey's post hoc test.