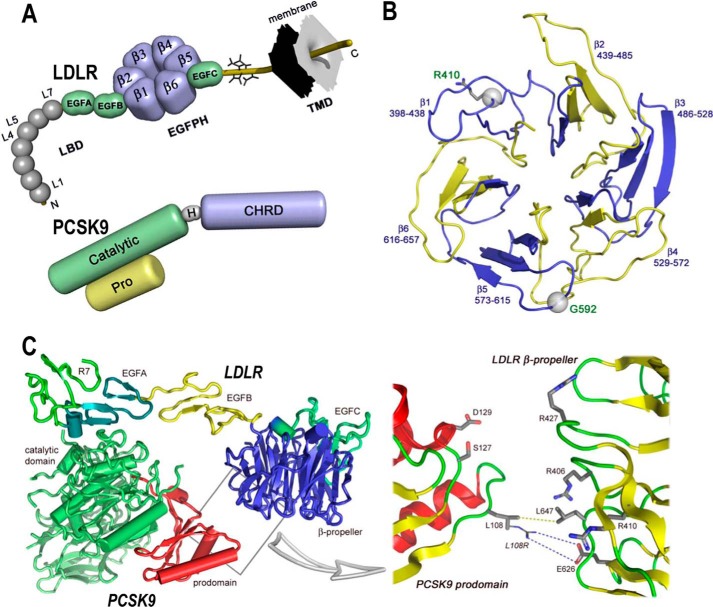
FIGURE 1.
Domain organizations of LDLR and PCSK9 and structures of LDLR β-propeller domain and of PCSK9·LDLR complex. A, domain organizations of LDLR (top) and PCSK9 (bottom). LDLR is composed of the ligand binding domain (LBD), containing seven repeats (L1–L7) of ∼40 residues, the EGF precursor homology domain (EGFPH), consisting of EGFA, EGFB, six-bladed (1–6) β-propeller, and EGFC domains, the O-linked sugar regions, and the transmembrane domain (TMD). Mature PCSK9 is formed after auto-cleavage between the prodomain (Pro) and the catalytic domain (Catalytic). The hinge domain (H) and the C-terminal histidine-rich domain (CHRD) are indicated. B, LDLR β-propeller domain with its six blades and their corresponding residues. The positions of Arg410 and G592 in the β-propeller domain of the LDLR are displayed. C, overall structure of the PCSK9·LDLR complex (PDB code 3M0C) shows two interfaces of interaction between the two proteins as follows: a major interface between catalytic domain of PCSK9 (green) and the EGFA domain of LDLR (turquoise) and a minor interface (boxed) between the prodomain of PCSK9 (red) and the LDLR β-propeller domain (blue). Box, details of the prodomain/β-propeller interface; yellow dotted line shows the van der Waals interaction between Leu108 (PCSK9) and Leu647 (LDLR-WT), whereas the blue dotted line depicts the “putative” ionic interaction between the GOF mutation L108R (PCSK9) and Glu626 (LDLR-WT).