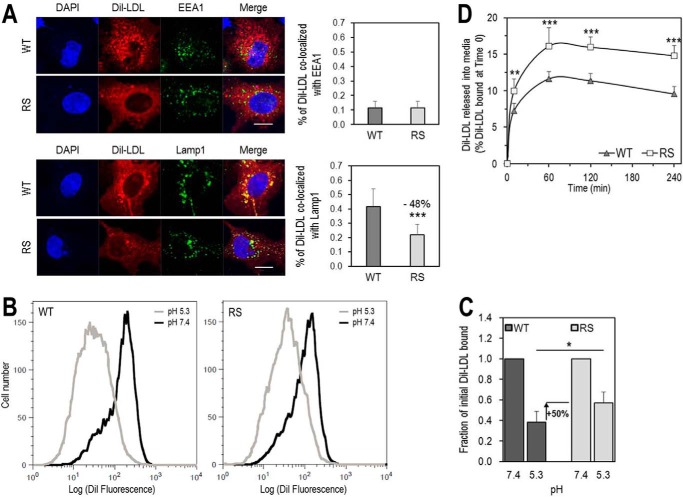
FIGURE 9.
Residue Arg410 of the LDLR is important for LDL release in acidic early endosomes and for its delivery to late endosomes. A, HepG2 cells overexpressing LDLR-WT (WT) or LDLR-R410S (RS) were incubated with DiI-LDL (5 μg/ml) for 4 h at 37 °C and analyzed by immunofluorescence under permeabilized conditions. Upper panels, co-localization of DiI-LDL with markers for early endosomal compartment (EEA1) and quantification. Lower panels, co-localization of DiI-LDL with markers for late endosomal/lysosomal compartment (Lamp1) and quantification. Quantifications were derived from analyses of 12 transfected cells (EGFP-positive)/condition/experiment. Scale bar, 15 μm. B and C, HEK293 cells overexpressing WT or RS were incubated for 1 h at 4 °C with 15 μg/ml DiI-LDL at pH 7.4, washed, and switched to pH 5.3 for an additional hour at 4 °C. At each pH, the amount of fluorescence from DiI-LDL bound to each receptor was measured by FACS. B, flow cytometry plots for WT (left) and RS (right). C, quantification of DiI-LDL bound at each pH relative to pH 7.4. D, HEK293 cells overexpressing WT or RS were incubated with DiI-LDL (6 μg/ml) for 1 h at 4 °C, washed, and then switched to fresh serum-free DMEM at 37 °C for the indicated times. DiI-LDL released into media was measured and reported relative to DiI-LDL bound at time 0. Data are averages ± S.D. of at least three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (t test).