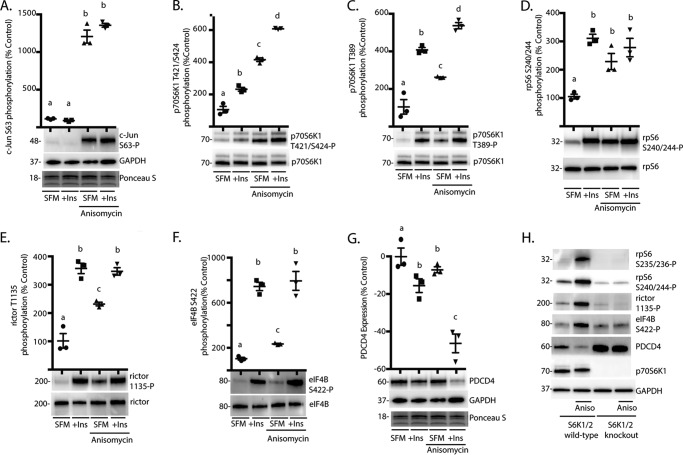
FIGURE 2.
The JNK agonist anisomycin promotes p70S6K1 phosphorylation and activation of the kinase. HEK293E cells in culture were maintained in DMEM supplemented with 10% FBS and serum-deprived (SFM) for 2 h to repress phosphorylation of S6K1. During the last 30 min of serum deprivation, cells were treated with anisomycin (20 μm) and/or insulin (Ins; 100 nm) as indicated. Phosphorylation of c-Jun on Ser-63 (A), p70S6K1 on Thr-421/Ser-424 (B) and Thr-389 (C), rpS6 on Ser-240/244 (D), rictor on Thr-1135 (E), and eIF4B on Ser-422 (F) were assessed by Western blotting. G, expression of PDCD4 relative to GAPDH was also assessed by Western blotting. Protein loading was assessed by Ponceau S. Values are the means ± S.E. Statistical significance is denoted by the presence of different letters above each scatter plot on the graphs. Scatter plots with different letters are statistically different; p < 0.05. Results shown in A–G are representative of two independent experiments; within each experiment, three independent samples were analyzed. H, wild-type and S6K1/2 knock-out MEF were serum-deprived for 2 h and then treated with anisomycin (Aniso) as described above. Phosphorylation of rpS6 on Ser-235/236 and Ser-240/244, rictor on Thr-1135, and eIF4B on Ser-422 as well as expression of PDCD4, p70S6K1, and GAPDH were assessed by Western blotting. Blots shown in H are representative of three experiments; within each experiment, four independent samples were analyzed. Protein molecular mass in kDa is indicated at the left of the blots.