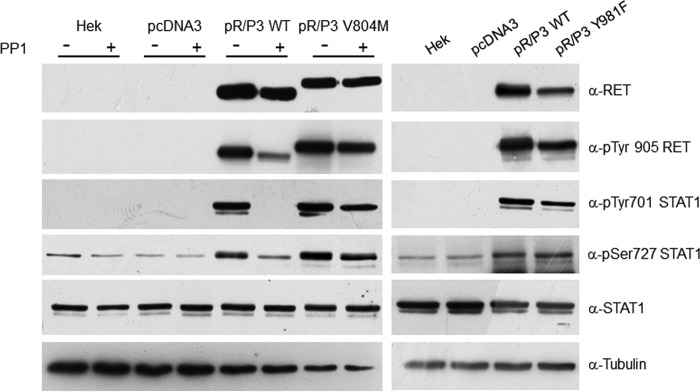
FIGURE 8.
Src does not mediate RET/PTC3-induced activation of STAT1. HEK293T cells, transiently transfected with pcDNA3, pcDNA3-RET/PTC3 WT, and the mutant pcDNA3-RET/PTC3-V804M Myc-tagged, were treated with the Src inhibitor PP1 (5 μm) for 24 h and harvested for cell lysate preparation. Immunoblotting for RET, pTyr-905 RET, pTyr-701 STAT1, pSer-727 STAT1, total STAT1, and tubulin were performed. Treatment with PP1 caused a marked reduction of RET Tyr-905 and STAT1 Tyr-701 phosphorylations in cells transfected with RET/PTC WT (pR/P3 WT), but not in cells transfected with the mutant RET/PTC3-V804M (pR/P3 V804M), which is resistant to PP1. Furthermore, HEK293T cells were transiently transfected with pcDNA3, pcDNA3-RET/PTC3 WT, and the mutant pcDNA3-RET/PTC3-Y918F, which is unable to bind Src, and harvested for cell lysate preparation, 24 h after transfection. Immunoblotting for RET, pTyr-905 RET, pTyr-701 STAT1, pSer-727 STAT1, total STAT1, and tubulin were performed. In cells transfected whit RET/PTC3-Y918F (pR/P3 Y918F) phosphorylation of STAT1 Tyr-701 was superimposable to that of RET/PTC3 WT (pR/P3 WT). These results indicate that Src is not involved in RET/PTC3-induced STAT1 Tyr-701 phosphorylation. All the experiments were repeated twice and the more representative results are depicted.