FIGURE 9.
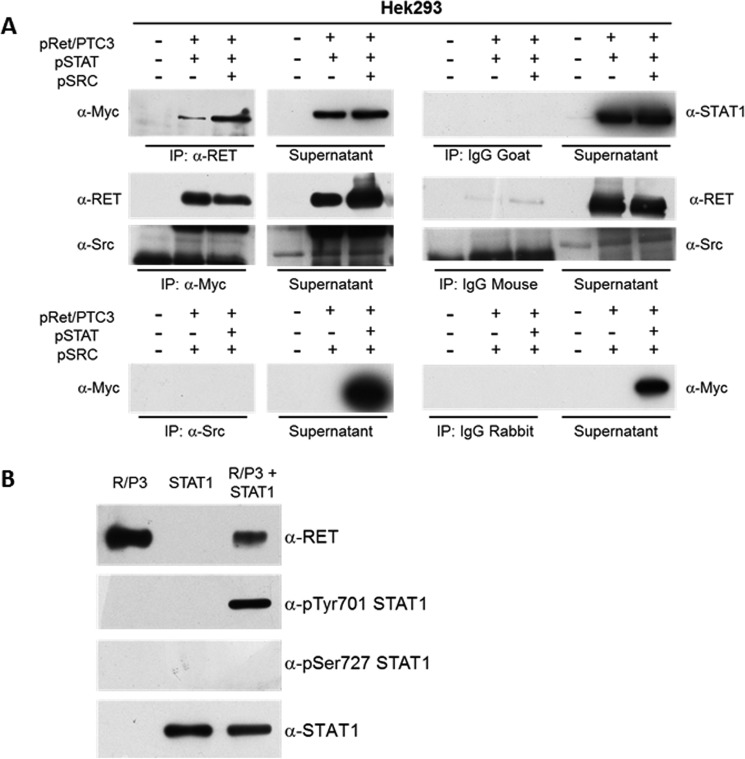
RET/PTC3 interacts with STAT1 and phosphorylates STAT1 on Tyr-701. A, co-immunoprecipitation experiment in HEK293T cells. HEK293T cells were transiently co-transfected with expression vectors for RET/PTC3 and Myc-tagged STAT1 or RET/PTC3 and Src or RET/PTC3, Myc-tagged STAT1 and Src. Twenty-four hours after transfection, cells were harvested for cell lysate preparation. Five hundred μg of total lysate were immunoprecipitated (IP) using an anti-RET antibody or an anti-Myc antibody (used for STAT1 IP) or an anti-Src antibody. Immunoprecipitates were then subjected to Western blotting using the anti-Myc antibody in RET IP, the anti-RET and anti-Src antibodies in STAT1 IP, and the anti-Myc antibody in Src IP. To verify binding specificity, the presence of STAT1, RET, and Src was searched on the protein complexes immunoprecipitated with normal goat (control of RET IP), normal mouse (control of Myc IP), or normal rabbit (control of Src IP) IgG mixtures. Immunoprecipitation of RET allowed a specific co-immunoprecipitation of STAT1 in the cells expressing RET/PTC3 and STAT1 or RET/PTC3, STAT1, and Src. Similarly, immunoprecipitation of STAT1 (with an anti-Myc antibody) allowed a specific co-immunoprecipitation of RET in the cells expressing RET/PTC3 and STAT1 or RET/PTC3, STAT1, and Src. Conversely, Src was not detected in the STAT1 IP and STAT1 could not be found in Src IP. These data indicate that STAT1 and RET/PTC3 aggregate one with the other, whereas Src and STAT1 do not. B, RET/PTC3 in vitro kinase assay. HEK293T cells were transiently transfected with expression vector for RET/PTC3 or Myc-tagged STAT1. Twenty-four hours after transfection, cells were harvested for cell lysate preparation. Five hundred μg of total lysate were immunoprecipitated using an anti-RET antibody or an anti-Myc antibody (used for STAT1 IP). The two IPs were pulled together and resuspended in kinase buffer. The reaction was carried out for 30 min at 30 °C and stopped by adding Laemmli buffer. The kinase reactions were submitted to Western blotting and the presence of RET, pTyr-701 STAT1, pSer-727 STAT1, and total STAT1 were assayed using the specific antibodies. RET/PTC3 phosphorylated STAT1 Tyr-701, but not STAT1 Ser-727. Furthermore, RET/PTC3 and STAT1 were incubated alone in the kinase buffer. No evidence of RET/PTC3-driven pulldown of phosphorylated STAT1 from HEK293T cell lysates or STAT1 autophosphorylation could be detected. All experiments were repeated twice and the more representative results are depicted.