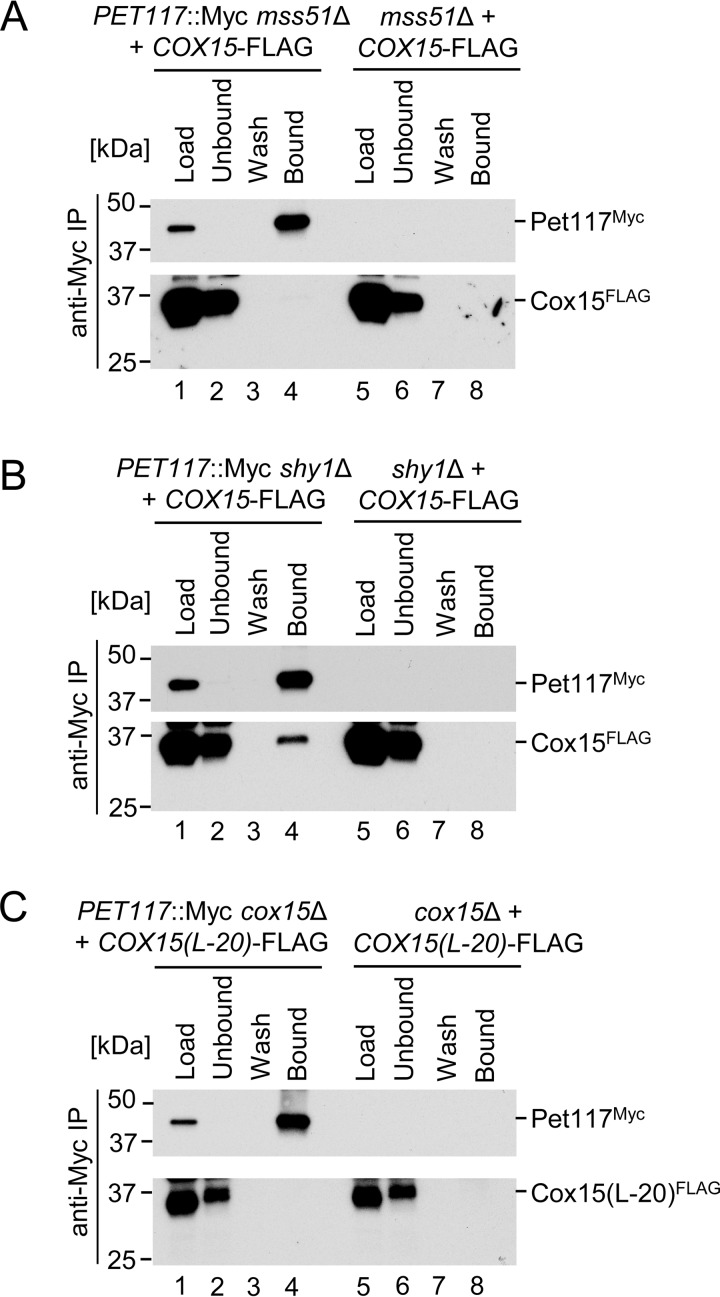
FIGURE 7.
Pet117-Cox15 physical interaction is dependent upon the presence of Mss51 but not Shy1 and is lost in an oligomerization-deficient Cox15 mutant. A, co-immunoadsorption of differentially tagged Pet117 and Cox15 in the absence of Mss51. Digitonin-solubilized mitochondrial lysates from mss51Δ or PET117::Myc mss51Δ cells expressing Cox15-FLAG were analyzed as in Fig. 5D. Results are shown for one experiment, representative of three independent experiments (biological replicates). The positions of molecular mass markers are indicated to the left of the immunoblots. B, co-immunoadsorption of differentially tagged Pet117 and Cox15 in the absence of Shy1. Digitonin-solubilized mitochondrial lysates from shy1Δ or PET117::Myc shy1Δ cells expressing Cox15-FLAG were analyzed as in Fig. 5D. Results are shown for one experiment, representative of three independent experiments (biological replicates). The positions of molecular mass markers are indicated to the left of the immunoblots. C, co-immunoadsorption of differentially tagged Pet117 and a variant of Cox15 lacking the matrix-exposed 20-amino acid region linking the two heme-binding domains of Cox15. Digitonin-solubilized mitochondrial lysates from cox15Δ or PET117::Myc cox15Δ cells expressing Cox15(L-20)-FLAG were analyzed as in Fig. 5D. Results are shown for one experiment, representative of three independent experiments (biological replicates). The positions of molecular mass markers are indicated to the left of the immunoblots.