FIGURE 5.
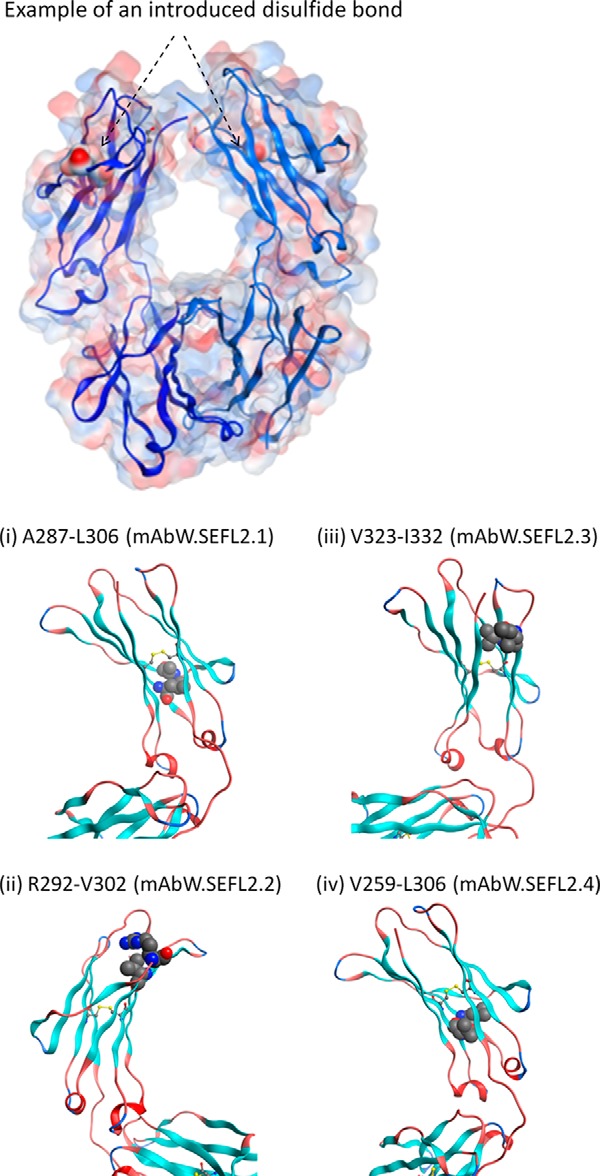
Top panel, transparent surface representation of Fc with an example of an introduced disulfide bond. The residue pair (Ala287-Leu306) that is mutated to Cys are shown in solid surface representation with an arrow indicating its location. Note that Fc is a homodimer. Bottom panel, ribbon representation of the CH2 domain showing the predicted 4 sites for engineering the disulfide bond to improve stability. The sites were identified based on structural parameters such as distance between Cα-Cα and Cβ-Cβ atoms (described in Ref. 30), solvent exposure (prefer buried sites), secondary structure (avoid loops), and away from FcRn binding sites. The Fc and CH2 domain coordinates are derived from Protein Data Bank entry 1L6X, a high resolution (1.65 Å) crystal structure of Fc fragment of Rituximab (39). The disulfide modeling as well as the generation of figures shown here were carried out using the Molecular Operating Environment software (Chemical Computing Group Inc.).
