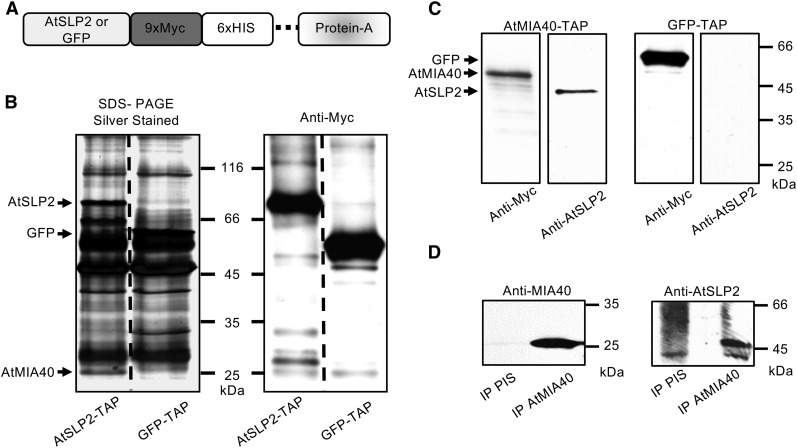
Figure 1.
AtSLP2-TAP pull-downs isolate AtSLP2-specific protein interactors. A, Cartoon depiction of the C-terminal TAP tag in frame with either AtSLP2 or GFP. The dashed line represents a human rhinovirus 3C protease cleavage site. B, Representative silver-stained SDS-PAGE and anti-Myc immunoblot of TAP pull-downs from stably transfected dark-grown Arabidopsis cell culture. GFP-TAP and AtSLP1-TAP pull-downs offered a control for nonspecific protein interactors (Supplemental Table S1 and raw data deposited at massive.ucsd.edu). Lanes contain 30 µL (silver) and 5 µL (western) of TAP-purified protein from 50 g of dark-grown Arabidopsis cell culture. C, Reciprocal AtMIA40-TAP pull-downs verify specific interaction between AtSLP2 and AtMIA40. AtMIA40-TAP was stably transfected into dark-grown Arabidopsis cell culture and used as bait to pull-down endogenous partners, which included AtSLP2. AtSLP2 was absent from in-parallel GFP-TAP pull-downs. D, Immunoprecipitation of endogenous AtMIA40-AtSLP2 protein complex using affinity-purified anti-AtMIA40 IgG and preimmune serum IgG (PIS). Immunoblotting was performed using 0.5 µg/mL anti-Myc (ICL), 1:1,000 anti-AtMIA40, and 1.2 µg/mL affinity-purified anti-AtSLP2 (Uhrig and Moorhead, 2011).