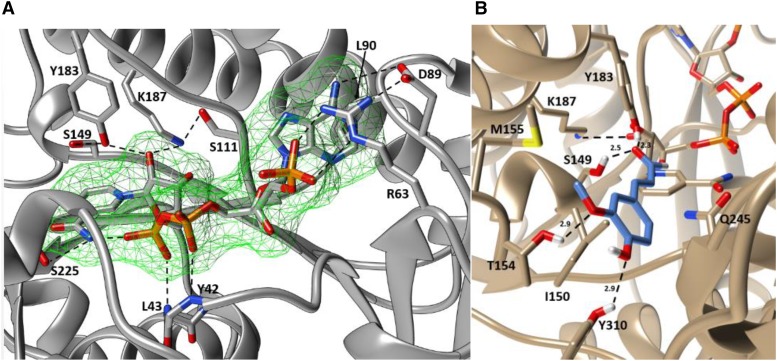
Figure 2.
A, The observed NADP+ in the binding pocket of SbCCR1. NADP+ and all interacting residues are represented as stick models. The backbone of SbCCR1 is represented as a ribbon diagram, and dashed lines represent hydrogen bonds or ionic interactions. All residues that contribute to NADP+ binding are labeled according to their single-letter abbreviations and numbered according to sequence positions. The catalytic triad, composed of Ser-149, Tyr-183, and Lys-187, is in close proximity to the nicotinamide ring and serves to promote hydride transfer to hydroxycinnamoyl-CoA substrates. B, Coniferaldehyde docked into the putative phenylpropanoid-binding region of SbCCR1. The backbone of SbCCR1 is represented by a ribbon diagram, with protruding side chains that contribute to coniferaldehyde binding modeled as sticks. Coniferaldehyde, which is the product of the reaction with the preferred substrate feruloyl-CoA, is shown in gray. Kinetics experiments with T154A and Y310F mutants revealed that these two residues are critical for binding the phenylpropanoid portion of feruloyl-CoA. Molecular graphics images were produced using the UCSF Chimera package.