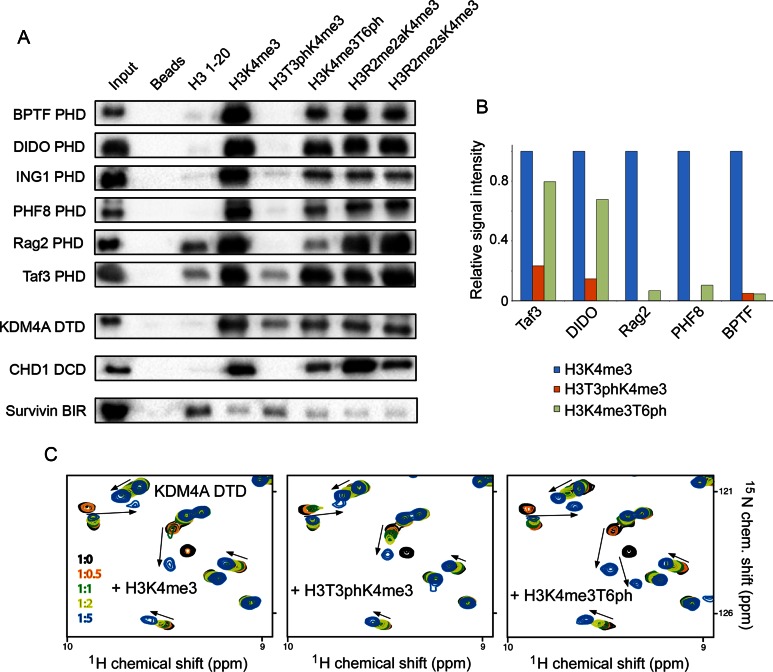
Figure 5.
The phospho/methyl switching mechanism is conserved. (A) Peptide pulldown experiments comparing the binding of readers to the indicated H3 peptides (residues 1–20). R2me2s and R2me2a are symmetrically and asymmetrically, respectively, dimethylated arginine 2. All peptides were biotinylated at the C-terminus and were preincubated with streptavidin-coated beads prior to addition of reader domains. Beads without peptide served as the negative control. (B) Binding of readers to the indicated peptides was probed by peptide microarrays. Signal intensity was normalized to the signal intensity for binding to H3K4me3 peptide. (C) Superimposed 1H,15N HSQC spectra of DTD of KDM4A collected upon titration with indicated peptides. Spectra are colour coded according to the protein:peptide molar ratio (inset).