Figure 6.
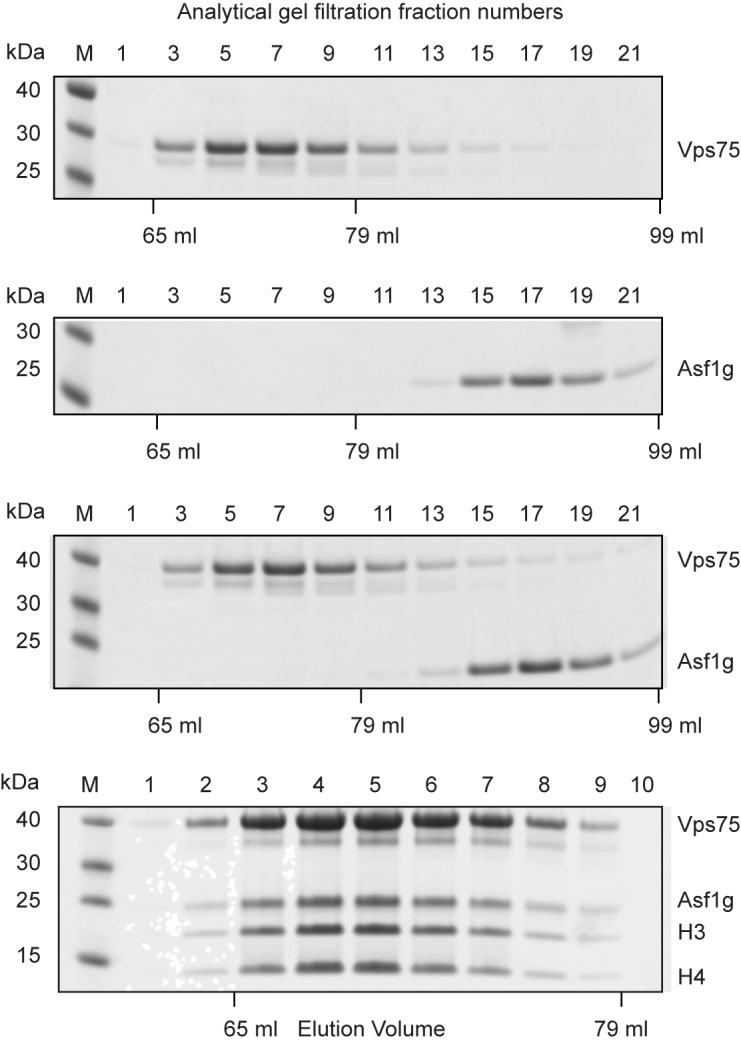
Vps75 binding to H3–H4 is compatible with Asf1 binding. SDS-PAGE analysis (4–12% Bis–Tris NuPAGE gels, Invitrogen) of fractions from the analytical gel filtration performed on a preparative scale using a HiLoad 16/600 Superdex 200 column (GE Healthcare). To span the peaks of Vps75 and Asf1, every other 2 ml fraction was analysed by SDS-PAGE as reflected by their fraction numbers (fractions 1–21). In contrast each fraction of the eluting Vps75–Asf1–H3H4 complex is shown (fractions 1–10). Vps75 does not interact with Asf1g in the absence of histones but can form a co-chaperone complex in the presence of histone H3–H4. M = PageRuler Prestained Protein Ladder (Thermo Scientific).
