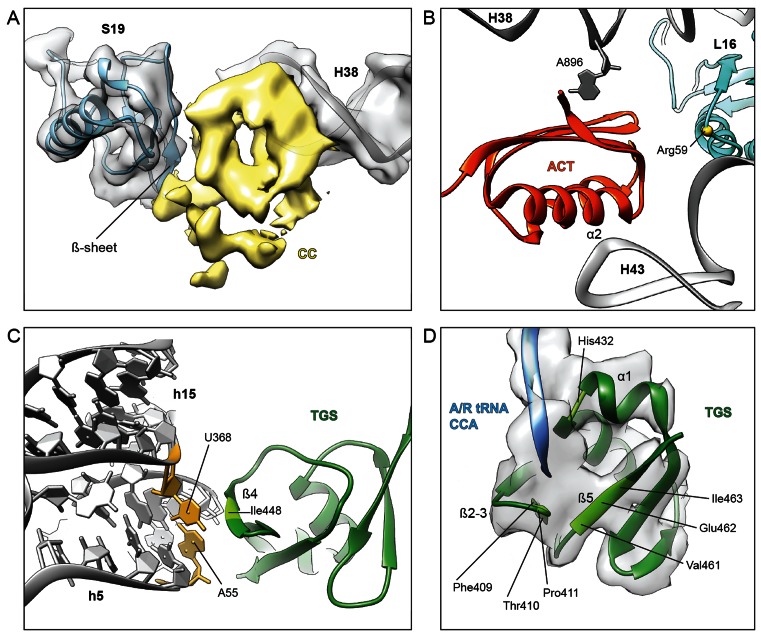
Figure 4.
Interactions of RelA with the ribosome and A/R-tRNA. (A) Cryo-electron density of the CC subdomain of RelA (yellow) suggests interaction with the β-sheet of r-protein S19 (blue) and the minor groove of H38 in the 23S rRNA (grey). (B) Interaction of the C-terminal RelA ACT domain with Arg59 of r-protein L16 (blue) as well as 23S rRNA residues A896 of H38 and the tip of H43. (C) The β4-strand of the TGS domain of RelA (green) approaches the minor groove of h5 of the 16S rRNA, where residues in the vicinity of Ile448 (light green) appear to interact with the base-pair formed by nucleotides A55 and U368 (yellow), which are flipped-out of helices h5 and h15, respectively. (D) Tentative placement of the A/R-tRNA CCA-end (blue) shows vicinity of C74 of the A/R-tRNA with His432 located at the kink in α-helix α1, whereas C75 and A76 enter into a pocket formed by the β1–β2 hairpin and β5-strand of TGS (green).