Key Points
Hodgkin lymphoma microenvironment T-helper cells express TH1/activation markers and lack TH2/immunosuppression markers.
These cells are functional, retaining the capacity for cytokine secretion and proliferation in vitro.
Abstract
CD4+ T-helper cells (THs) dominate the classical Hodgkin lymphoma (CHL) microenvironment, but their role is poorly understood. Advances in flow cytometry and immunohistochemistry permit more detailed investigation of this aspect of CHL pathophysiology. To address the hypothesis that the TH-infiltrate, rather than being TH2-enriched, senescent and hypofunctional, is TH1 and activation marker-rich, cytokine-secretory and proliferative, we applied comprehensive flow cytometric immunophenotyping and functional assays of cytokine secretion/proliferation to TH cells from 18 CHL-derived single-cell suspensions (SCSs) compared to reactive lymph nodes (RLNs). CHL-derived TH cells express TH1-associated CXCR3/CCR5 and TNFα/IFNγ/interleukin-2 (IL-2) and less TH2-associated CCR3/CCR4, with no IL-4/IL-13. They lack exhaustion-/suppression-associated PD1, CD57 and terminally differentiated effector memory cells, with more central memory cells, activation-associated partners of Hodgkin Reed Sternberg (HRS) cell-expressed CD30/OX40-L/ICOS-L, and other activation markers. TH cell lines established from CHL and RLN-derived SCSs remain cytokine-secretory. We confirmed and extended these studies using tissue microarray immunohistochemistry (TMA-IHC) from a large CHL tissue bank (n = 122) and demonstrate TH1-associated TBET is abundant in CHL, and TH2-associated CMAF/GATA3 and exhaustion-associated PD1 expressed at significantly lower levels. These molecular insights into the CHL-associated TH offer potential diagnostic, prognostic and pharmacologically modifiable therapeutic targets and do not support the established view of a TH2-enriched, senescent/exhausted, hypofunctional, hypoproliferative infiltrate.
Introduction
The bulk of the cellular immune infiltrate in classical Hodgkin lymphoma (CHL) comprises CD4+ T cells (T-helper cells [TH]), along with cytotoxic T cells, macrophages, nonmalignant B cells, and innate immune cells.1 Antitumor immunity is largely T cell mediated,2 but the mechanisms underlying the failure of this rich T-cell infiltrate to clear the malignancy are poorly understood. A cell-mediated immune response orchestrated by TH1-polarized inflammation is likely central to tumor eradication,3,4 perhaps partly by suppressing a competing protumoral5 TH2-mediated response.6 Previous studies have suggested that in CHL, the TH-cell compartment is TH2 and regulatory T cell (Treg) enriched, senescent/exhausted, hypofunctional, and hypoproliferative,1 providing some explanation for the failed immune response. Systemic T-cell–specific immune defects are well described in CHL,7,8 but the complexity and heterogeneity of TH function challenges our understanding of their specific role in the microenvironment.
Previous reports showed that the lymphoid immune infiltrate of CHL is resistant to proliferation and cytokine secretion in vitro,9 suggesting a predominance of senescent/anergic TH cells. T cells expressing PD1 are functionally immunosuppressed through interaction with ligands PD-L1/210; however, evidence for PD1 expression in the microenvironment of CHL is limited despite substantial evidence that PD-L1 is expressed by the malignant Hodgkin Reed Sternberg (HRS) cell.11 Although one study found PD1 expressed on CD4+ T cells derived from CHL lymph nodes, it is based on just 3 patients,12 and other studies have found only extremely low expression levels.13-16 There are several other well-characterized markers of senescent TH cells, including the terminally differentiated effector memory cell (TEMRA), but no detailed investigation of memory subsets has been carried out. CD57, associated with chronic viral infection and impaired function,17 is well known to be underexpressed in CHL.15
In fact, there is already a limited evidence base that an activated, TH1-biased, rather than suppressed, TH2-biased infiltrate, could be a major component of the tumor microenvironment. TH1 cells are characterized by TBET expression and interleukin-2 (IL-2)/IFNγ/TNFα production, and TH2 by GATA3/CMAF expression and IL-4/IL-13/IL-21 production.18 TH1-associated TBET was found to be expressed to a greater extent than TH2-associated GATA3 in a small IHC study,19 although another study20 produced contradictory evidence, finding more TH2-associated CMAF expression than TBET. Several groups have found CHL-infiltrating lymphocytes to be cytokine secretory, capable of producing proinflammatory, TH1-biased cytokines such as IL-2, IFNγ, and TNFα as well as TH2 and immunosuppressive cytokines.21-24 TH1/TH2 polarization is also suggested by chemokine receptor (CCR) profiling, with CCR3 and CCR4 preferentially expressed by TH2-polarized cells25 and CXCR3 and CCR5 by TH1-polarized cells.26 Although TH2-associated CCR3 and CCR4 are receptors for the CHL-associated chemokines Regulated upon Activation Normal T cell Expressed and Secreted/CCL5 (RANTES) and CCL17/thymus- and activation-regulated chemokine, respectively,27,28 RANTES may also bind TH1-associated CCR5, and CCR4 is also a marker of Tregs, hence limiting the interpretation of TH polarization data based solely on CCR expression. However, previous publications have shown a significant proportion of infiltrating TH cells express TH1-associated CXCR3 and CCR5.29,30 TH2-associated CCRs CCR3 and CCR4 were also demonstrated in the lymphoid microenvironment in these studies, but not to a greater extent than the TH1-associated CCRs. These studies incorporated small numbers of samples and were limited to isolated investigations of only cytokine, CCR, or transcription factor expression but provide an evidence base supporting an activated TH1-rich microenvironment for further investigation in larger, more extensive cohorts.
An activated TH microenvironment may itself contribute to disease pathogenesis. The HRS cell is derived from a germinal center B cell with JAK/STAT and nuclear factor-κB pathway overexpression essential to its survival. Having lost of much of its B-cell phenotype, notably surface immunoglobulin, through which prosurvival signals would normally be delivered, the HRS cell is reliant upon intrinsic genetic defects and signaling through a range of overexpressed surface molecules, including LMP1 in Epstein Barr virus (EBV)-positive cases, and members of the tumor-necrosis factor and immunoglobulin receptor superfamilies, including CD70, CD80/CD86, CD30, CD40, OX40-L/CD252, and ICOS-L/CD275 (for a review, see Kuppers31). The reciprocal molecules, receptor, or ligand (CD27, CD28, CD153, CD154, CD134, and CD278, respectively) are often present on CHL microenvironment immune cells1 and all are upregulated on activated TH cells. CD27 and CD28 are expressed at high levels on unstimulated or naïve T cells, loss of expression associated with activation, and retention of CD27 expression associated with a memory cell phenotype.32 Specific expression of these molecules by CHL-infiltrating TH cells has not previously been comprehensively investigated.
New tools in tissue microarray immunohistochemistry (TMA-IHC) and multicolor fluorescence flow cytometric immunophenotyping (flow) and the discovery of TH-defining transcription factors, cell surface molecules and cytokine secretory profiles have enabled more extensive investigation of functional subsets in the malignant microenvironment, overcoming the limitations of previous studies. We used diagnostic frozen single-cell suspensions (SCSs) for flow, cytokine-secretion, and proliferation assays and a large tissue bank of formalin-fixed, paraffin-embedded (FFPE) tissue for TMA-IHC to characterize the TH infiltrate of CHL. We found a predominance of TH1 over TH2, an absence of markers of senescence, an excess of central memory cells (CMs), and retained cytokine secretory and proliferative capacity for CHL-associated T cells, with some markers showing associations with clinical outcome. These findings challenge the established view of a TH2-enriched, senescent, hypoproliferative infiltrate, provide insight into molecular interactions between functionally active TH cells and malignant HRS cells, and offer potential diagnostic, prognostic, and pharmacologically modifiable therapeutic targets.
Methods
Ethics approval was obtained from the local regional ethics committee. All patient-derived samples were obtained with informed consent in accordance with the Declaration of Helsinki and stored under conditions compliant with the Human Tissue Act 2008.
Patient samples for frozen SCSs
SCSs derived from surplus material available after diagnostic biopsy were retrieved from nitrogen-frozen archived samples, with patient, histological, and storage characteristics detailed in Table 1 (CHL: n = 18; reactive lymph node [RLN]: n = 6). Details of storage/retrieval methods and selection of control samples are provided in the supplemental Methods. The median age of patients with CHL (29 years, range 16-81) and those with reactive nodes (24 years, range 18-73) was not significantly different (P = .713). The median duration of time for which SCSs derived from CHL were held in storage (48 months, range 11-149) was significantly longer (P = .024) compared with that from reactive nodes (18 months, range 5-23). However, no correlation between sample age and expression of any single marker by flow could be demonstrated (data not shown).
Table 1.
Summary of patient characteristics for the immunophenotype study
| Histology | Age at biopsy (y) | Node origin | Sample age (mo) | EBV status |
|---|---|---|---|---|
| CHL (LR) | 35 | Cervical | 81 | + |
| CHL (MC) | 16 | Cervical | 149 | + |
| CHL (MC) | 31 | Cervical | 75 | + |
| CHL (MC) | 33 | Cervical | 49 | + |
| CHL (NK) | 23 | NK | 139 | NK |
| CHL (NS) | 38 | Cervical | 44 | − |
| CHL (NS) | 20 | Cervical | 82 | − |
| CHL (NS) | 28 | Cervical | 11 | − |
| CHL (NS) | 45 | Axilla | 31 | − |
| CHL (NS) | 23 | Cervical | 39 | − |
| CHL (NS) | 31 | Supraclav | 44 | − |
| CHL (NS) | 21 | Inguinal | 35 | − |
| CHL (NS) | 81 | Cervical | 119 | − |
| CHL (NS) | 24 | Cervical | 80 | − |
| CHL (NS) | 28 | Cervical | 149 | + |
| CHL (NS) | 40 | Cervical | 44 | + |
| CHL (NS) | 29 | Cervical | 46 | + |
| CHL (NS) | 18 | Cervical | 18 | NK |
| FH | 19 | Cervical | 23 | NA |
| FH | 68 | Cervical | 18 | NA |
| FH | 18 | Submental | 17 | NA |
| PH | 21 | Cervical | 18 | NA |
| PH | 73 | Cervical | 22 | NA |
| PH | 27 | Axilla | 5 | NA |
EBV status defined by LMP1 or EBER depending upon era of biopsy.
Median age patient (years) = 29/24/27. Median age SCS sample CHL/Reactive/PH (mo) = 48/18/18.
FH, follicular hyperplasia; LR, lymphocyte rich; MC, mixed cellularity; NK, not known; NS, nodular sclerosis; PH, paracortical hyperplasia.
Flow
Flow was carried out using standard methods for fluorescence immunophenotyping and intracellular cytokine stimulation and staining, with details of fluorochrome-conjugated antibodies and reagents provided in the supplemental Material.
In vitro cell culture
SCS-derived cells were cultured based on a technique developed for proliferating tumor-infiltrating lymphocytes33 but optimized using conditions and a cytokine combination found best to support long-term TH growth, as detailed in the supplemental Methods.
Patient samples for TMA construction and immunohistochemistry
We identified 122 adult patients of known clinical outcome diagnosed at St. Bartholomew’s Hospital who had high-quality, FFPE tissue from the original diagnostic biopsy available, the characteristics of which were previously published and are summarized in Table 2.12 Median follow-up was 16.5 years (range, 2-40 years). TMA construction and quality control, immunohistochemistry, EBV status, and automated cell counting were carried out as described in the supplemental Methods and as previously published.34
Table 2.
Summary of patient characteristics for the TMA-IHC study
| Total number of patients in original cohort (n = 122) | Percent of original cohort (n = 122) | |
|---|---|---|
| Male | 79 | 65 |
| Age >45 y | 27 | 22 |
| Advanced (stage IIB-IV) | 87 | 71 |
| Anthracycline-based chemotherapy | 56 | 46 |
| Alkylator-based chemotherapy | 52 | 43 |
| Radiotherapy only | 14 | 11 |
| Combined modality | 48 | 39 |
| Histological subtype | ||
| Nodular sclerosis | 93 | 78 |
| Mixed cellularity | 25 | 20 |
| Classical lymphocyte rich | 0 | — |
| Lymphocyte deplete | 2 | 2 |
| EBER-ISH + | 38 | 31 |
Statistical analysis
All pairwise comparisons were carried out using the Mann-Whitney U test. For comparisons of markers detected using flow, we determined percentage of CD3+CD4+ cells expressing each marker and calculated median expression levels for CHL-derived CD3+CD4+ cells (CHL-TH; n = 18), paracortical hyperplasia-derived cells only (PH-TH, n = 3), and all RLN-derived cells comprising both PH-TH cells and follicular hyperplasia-derived TH cells (RLN-TH; n = 6). Statistically significant differences were determined using pairwise comparisons of medians for CHL-TH vs PH-TH and CHL-TH vs RLN-TH. Full results and statistical analysis are presented in supplemental Table 1. For TMA-IHC, median cell count per mm2 was calculated for each marker derived from all patient samples based on the automated image analysis cell count and expression levels for each marker compared pairwise. Pairwise comparisons were also carried out based on EBV status (positive or negative) and the major histological subtypes (nodular sclerosis or mixed cellularity).
Survival outcomes were measured from date of diagnosis to occurrence of event or date of last follow-up. These were: overall survival (OS); death from any cause; disease-specific survival (DSS); death from disease or treatment; and freedom from first-line treatment failure, the event being death, first relapse, or progression on first-line therapy. Each case was classified as high or low expression based on the number of microenvironment cells/mm2 expressing each marker. The cutpoint was generated for each marker by using the X-Tile statistical package35 (Yale University, New Haven, CT) with the test/validation set methodology, as detailed in the supplemental Methods. Kaplan-Meier curves defined by these cutpoints were generated and statistical significance of differences arising from differential expression of each marker determined using the log-rank test. Multivariate models were built using a Cox proportional-hazards regression model (forward stepwise likelihood ratio) incorporating variables selected with a univariate P value < .05. Statistical significance for all tests was set at .05.
Results
Extended immunophenotype of SCS-derived TH cells
Expression levels and statistical comparisons of all markers measured by flow are provided in supplemental Table 1. The relative composition of CD4, CD8, and CD20 was similar for SCS derived from CHL, PH, and all RLN (supplemental Figure 4).
TH1 and TH2
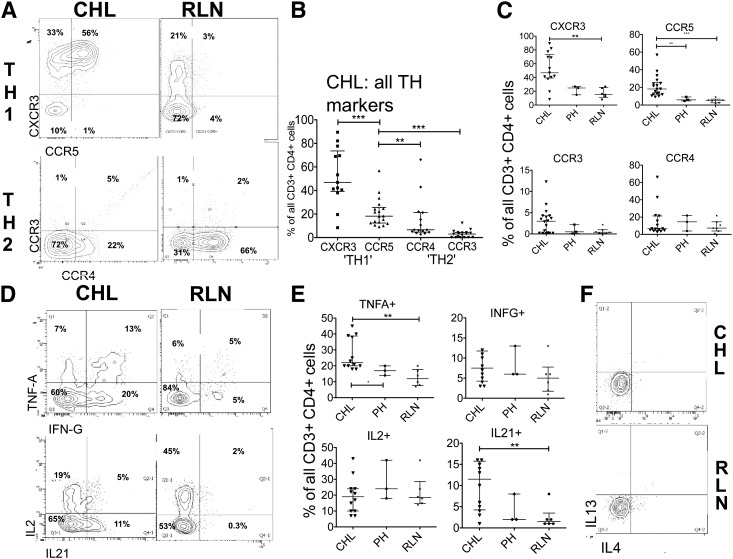
Because TH2 polarization is thought to contribute to the failed immune response in CHL, we first investigated TH1 and TH2 polarization in the CHL-TH. Using flow, we assessed TH1- and TH2-associated CCR expression in CHL (CHL-TH) and RLN (RLN-TH), including CCRs recognizing HRS cell-expressed chemokines CCL17/thymus- and activation-regulated chemokine and CCL5/RANTES. The median proportion of CHL-TH cells expressing the TH1-associated CCRs CXCR3 (45%) and CCR5 (22%) was significantly greater (P < .005) than expressing TH2-associated CCR3 (8%) or CCR4 (4%) (Figure 1A-B). Further, TH1-associated CCRs were more highly expressed in CHL-TH than in RLN-TH cells (Figure 1C), with similar proportions expressing TH2-associated CCRs. We used a cytokine stimulation assay to determine the functional capacity of TH cells to produce TH1- or TH2-defining cytokines. CHL-TH produced TH1 cytokines to a significantly greater (for TNFα) or comparable (for IFNγ and IL-2) extent compared with RLN-TH (Figure 1D-E) and although the TH2 cytokines IL-4 and IL-13 were detected in TH2-polarized cell lines, they were not detectable in SCS-derived TH cells from any primary source (Figure 1F). IL-21 (Figure 1E), a cytokine without clear TH subset-defining features, was expressed in a significantly greater proportion (P < .005) of CHL-TH cells (11%) compared with RLN-TH (1%).
Figure 1.
Expression of TH-polarization and CHL-associated CCRs as summarized in supplemental Table 1. (A) Representative flow plots and (B) summary of expression of TH1 and TH2 demonstrating a greater proportion of CHL-TH cells expressing TH1-related CCRs than TH2-related CCRs. (C) Greater expression of TH1-related CCRs in CHL-TH than in RLN-TH. (D) Representative flow plots and (E) summary of expression of TH-related cytokines measured by intracellular cytokine assay demonstrates greater or equivalent expression levels of TH1-related cytokines TNFα, IFNγ, and IL-2, along with IL-21 in CHL-TH compared with RLN-TH, with no measurable TH2-related cytokine expression (F). RLN comprised 6 samples, of which 3 were classified PH and 3 FH. Results for PH are additionally presented as a separate category in these figures for reasons described in the supplemental Material.
Senescence, immunosuppression, and memory
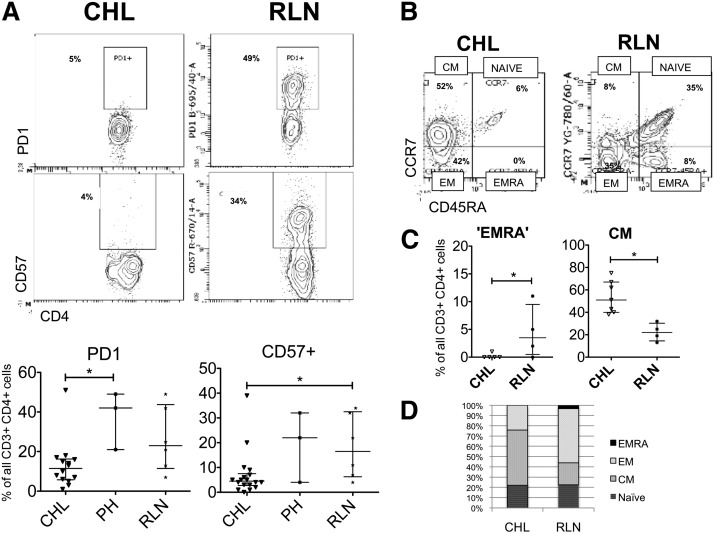
Because HRS cells overexpress PD-L1 and interaction with its T cell–expressed ligand PD1 is thought to contribute to the failed immune response in CHL, we next sought to determine the contribution of PD1 expression to immunosuppression in CHL-TH. PD1 was detectable only at low levels in CHL-TH cells and expressed in a higher proportion of RLN-TH cells (Figure 2A). We determined 2 other indicators of functional senescence in TH cells: the expression of CD57 and the TEMRA phenotype (CCR7-CD45RA+). CD57 was expressed in significantly fewer CHL-TH cells (4%) compared with RLN-TH (19%) (P < .050 (Figure 2A). The TEMRA phenotype was rarely found in CHL-TH cells, whereas it was detected in up to 10% of RLN-TH cells (Figure 2B). In fact, the dominant memory subset of CHL-TH cells was the CM, whereas for RLN-TH cells the dominant subset was the effector memory cell (Figure 2C-D). A similar pattern was found using the T-cell–homing selectin CD62L as a marker of central memory (not shown).
Figure 2.
Expression of markers of memory and senescence in TH cells. (A) Representative flow plots and summary of expression of PD1 and CD57 demonstrates relative absence of these markers of immunosuppression/senescence in CHL-TH compared with RLN-TH. (B) Representative flow plots and (C) summary of T memory subsets demonstrate a relative absence of TEMRA cells and increased proportion of CM cells in CHL-TH compared with RLN-TH. (D) The major differences in subset distribution between CHL-TH and RLN-TH, with bars divided according to the median proportion of cells defined by each memory phenotype and error bars omitted for clarity. Those subsets for which there are significant differences between CHL and RLN and presented with error bars in panel C.
Activation, Tregs, and proliferation
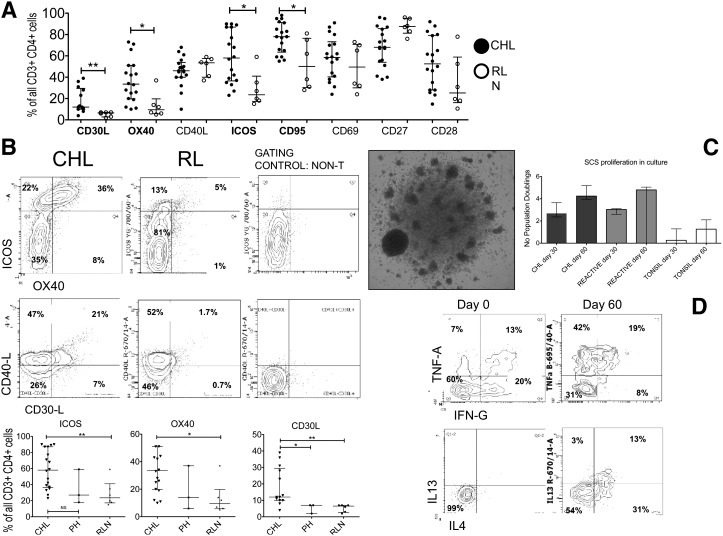
We find little evidence to support a TH2-skewed phenotype or evidence of excess senescence or exhaustion in CHL-TH compared with RLN-TH cells. We instead demonstrate that CHL-TH cells express high levels of TH1 cytokines and CCRs and CM markers at comparable or greater levels than in RLN-TH cells, suggesting an activated phenotype for the CHL-TH. We therefore stained for an extended panel of activation-modulated molecules, including receptor/ligands reciprocal for molecules expressed by the HRS cell (CD30, CD40, CD70, CD80/86, and CD134-L). These markers were all expressed by a high proportion of CHL-TH cells to a comparable or significantly greater extent than RLH-TH (Figure 3A-B). CD27 and CD28, both downregulated with stimulation, were expressed in a large proportion of CHL-TH cells; however, median CD27 expression levels were significantly lower than in RLN-TH.
Figure 3.
Expression of activation markers and proliferative capacity of TH cells. (A) Summary of all activation markers showing proportions of CHL-TH cells expressing all activation markers compared with RLN, with those showing statistically significant differences indicated. (B) Representative flow plots and summary comparing CHL-TH and RLN-TH expression levels for selected markers that showed increased median expression in CHL-TH compared with RLN-TH. Similar proportions of CHL-TH and RLN-TH expressed all other markers. (C) Representative photomicrograph (left, ×40 objective, Olympus BX61 microscope) and bar chart (right) summarizing proliferation CHL, RLN, and tonsil-derived SCSs at 30 and 60 days. (D) Representative flow plots showing capacity of proliferative cells to produce TH1 (TNFα and IFNγ, top) and TH2 (IL-4 and IL-13, bottom) cytokines at 60 days (right) compared with only TH1 at baseline (bottom) having been exposed to IL-2 and IL-4 in the culture medium.
Next, we investigated the expression of TH-associated markers of regulatory T-cell function, also nonspecifically upregulated on activated T cells (FOXP3, CTLA4, CD25/IL-2Rα). There is substantial evidence of a significant infiltrate of FOXP3-expressing cells in the CHL microenvironment proposed to represent regulatory T cells. Although we and others have previously demonstrated that high levels of FOXP3 expression are associated with good prognosis in CHL,34 there was no difference in median expression levels of FOXP3 compared with RLN-TH. We confirm that FOXP3 was expressed in CD4+ T cells coexpressing CD25 and CTLA4 (supplemental Figure 5). CTLA4 was expressed at significantly greater levels (P = .011) in CHL-TH (50%) than in RLN-TH (20%).
Given the activated, cytokine secretory, central memory-rich TH phenotype demonstrated in the previous experiments, we assessed the proliferative capacity of the TH cells. We optimized a culture system with IL-2/IL-4 supplementation to expand the TH population without antigen or mitogen. 15/18 CHL-derived SCS and 5/6 RLN-derived SCSs had adequate cells remaining after immunophenotyping for the cell culture experiment. There was no significant difference in proliferation between CHL- and RLN-derived culture systems (Figure 3C, median doublings in CHL- and RLN-derived systems, respectively, at day 30 = 2.7 vs 3.0 and day 60 = 4.2 vs 4.8; P = .67). Day 30 flow confirmed all culture systems to be predominantly comprised of TH-cells with ongoing cytokine-secretory capacity (Figure 3D). In contrast, tonsil-derived culture systems failed to proliferate.
Validation by TMA-IHC in a large independent cohort
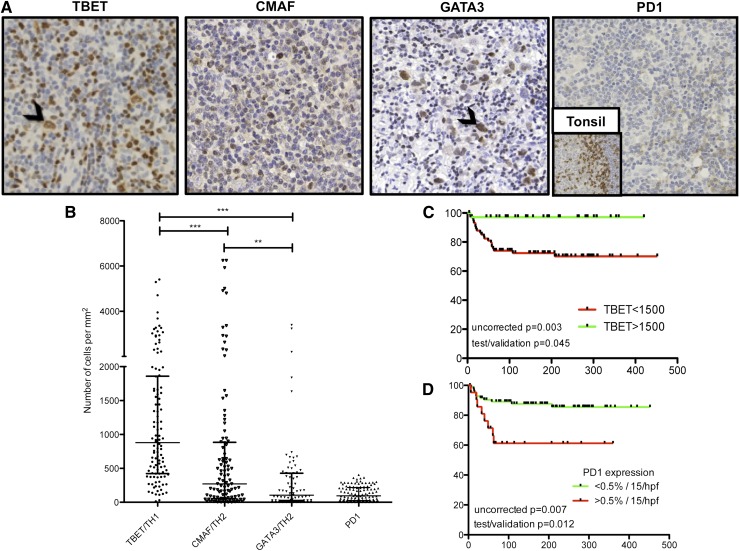
To corroborate our findings of TH1 predominance over TH2 and low expression levels of PD1 in situ without the histological architecture disruption and freeze-/thaw-induced changes of marker expression, we investigated a large independent cohort of FFPE patient samples. We used TMA-IHC to examine expression of TH1-associated TBET, TH2-associated CMAF/GATA3, and PD1 for 122 cases of CHL with known clinical outcome. Representative examples of cases are presented in Figure 4A. There was significant heterogeneity of expression between samples (Figure 4B). TBET was expressed in significantly greater numbers of microenvironment cells compared with CMAF (median 879 vs 272 cells/mm2, P < .0001) or GATA3 (105 cells/mm2, P < .0001). FOXP3 expression was previously described in this cohort,34 but notably was expressed in a higher number of cells than any other TH subset marker, including TBET (2346 vs 879 cells/mm2, P < .0001). Because a TH1-biased infiltrate may be expected to induce a more robust cytotoxic T-cell infiltrate, we tested for a correlation between TBET expression and number of CD8+ T cells by using data generated from previously published work.34 Interestingly, we found a significant positive correlation between numbers of CD8+ cells and numbers of TBET+ cells (Pearson R = 0.300; P < .005). There was no correlation between numbers of CD8+ cells and numbers of CMAF+ or GATA3+ cells (supplemental Figure 6).
Figure 4.
Immunohistochemistry to investigate TBET, CMAF, GATA3, and PD1 expression. Photomicrographs (×40 objective, Olympus BX61 microscope) of representative examples (A) showing median expression of TH1-associated TBET (extreme left, black arrowhead showing TBET+ HRS cell) and TH2-associated CMAF (center left) and GATA3 (center right, black arrowhead showing GATA3+ HRS cell). PD1 expression was undetectable in most cases with the example (extreme right) being one of the few cases with >15 cells/high-power field associated with adverse outcome. Expression is weak compared with the internal positive control of tonsil (inset). Numbers of cells expressing each TH-associated marker and PD1 are compared (B) showing TH1-associated TBET is expressed by greater numbers of cells compared with TH2-associated CMAF/GATA3 and with minimal PD1 expression. DSS curves indicate a positive impact of TBET expression (C) and a negative impact of PD1 expression (D) in univariate analysis.
We observed strikingly little expression of PD1 in the CHL microenvironment despite high levels seen in the internal positive control of tonsil (Figure 4A, far right and inset). There was no detectable PD1 expression in 42% of patients, and a further 40% showed expression in <0.5% of all nucleated cells.
Clinical associations
In light of the significant heterogeneity of expression of these markers and in order to explore the potential clinical impact of a TH1-polarized, activated TH infiltrate in contrast to a PD1-expressing suppressive infiltrate, we went on to assess clinical associations. This was only possible with the large number of samples available in TMA and hence only for those markers that were stained by IHC. Univariate survival analysis revealed associations between high levels of TBET expression and superior DSS (median 5-year DSS for >1500 cells/mm2 = 97% vs 77% for <1500 cells/mm2; test/validation set corrected P = .045) (Figure 4C), and with EBV status (1750 cells/mm2 for EBV+ vs 669 for EBV−, P = .0033; supplemental Figure 7) and between GATA3 and histological subtype, with microenvironment expression of GATA3 significantly greater in nodular sclerosis compared with mixed cellularity cases (183 vs 24 cells/mm2, P = .0028; data not shown). No other significant associations between transcription factor expression level, clinical outcome, EBV status, and histological subtype were found. Those patients having detectable PD1 expression (>15 cells/high-power field) had poorer 5-year DSS (63% vs 86%; P = .012), but OS (63% vs 84%, P = .18) was not significantly different (Figure 4D). Multivariate analysis incorporating only those markers expressed by the microenvironment that showed significant association with survival outcomes in univariate analysis in this and our previously published study34 (CD20, CD68, FOXP3, TBET, and PD1) was carried out (supplemental Table 8). Only high PD1 expression (P = .007) and low FOXP3 expression (P = .029) remained associated with adverse OS in this model. However, the limitations of multivariate analysis on incorporation of large numbers of variables with limited numbers of survival events are acknowledged, and hence any such analysis must be interpreted with caution.36 In those cases investigated by flow cytometry where single cell suspensions were available, we could not find any associations between EBV status and expression levels of markers. However, with only 9 EBV negative cases and 7 EBV positive (2 unknown), the sample size may have been underpowered.
Discussion
Advances in technology and in our understanding of markers of T-cell function have enabled us to revisit the phenotype of the CHL-infiltrating TH. This study is, to our knowledge, the most detailed investigation of the CD4+ TH in CHL using flow with corroboration in TMA-IHC. The TH1-associated CCRs CXCR3 and CCR5 were expressed in a greater proportion of CHL-TH cells than TH2-associated CCR3 and CCR4, and cytokine expression profiling indicates that the cells are TH1 polarized and proinflammatory without expression of TH2-associated cytokines. Compared with RLN-TH cells, the TH cells of the CHL microenvironment express more TH1-associated CCRs and produce TH1 cytokines to a similar or greater extent. IL-21 expression was notably restricted to CHL-TH cells. Although this cytokine has been described in TH2 cells, it has also been associated with diverse TH function, including TH1, TFH (follicular helper), TH17, memory, and enhancement of cell-mediated antitumor responses.37 There is also evidence that it is important for HRS cell survival.38 Although previous studies have suggested that IL-13 also appears to be a growth factor for HRS cells,39 we found no evidence of its production by CHL-TH cells, suggesting its activity only as an autocrine growth factor.
By TMA-IHC, we noted low expression of GATA3 or CMAF compared with levels of expression of TBET, where the presence of higher numbers of infiltrating cells was associated with EBV+ status and superior survival outcome. This association has been previously reported in pediatric CHL40 but never to our knowledge in an adult series. Associations between TBET and EBV status may be expected, with EBV infection inducing a TH1/cytotoxic T-cell response. A large gene expression profiling study of frozen whole tumor samples supports this hypothesis,41 where EBV+ cases showed overexpression of genes associated with TH1/cytotoxic immune response. However, TBET was not overexpressed in EBV+ cases in this unsorted whole-tumor gene expression profile. We acknowledge that expression of TH polarization-defining transcription factors has been described outside the context of a classical TH1/TH2 dichotomy. Expression of TBET has been shown in TH1 response-suppressing Tregs and immunoglobulin G2/3 class-switching B cells,42 GATA3 in T-cell precursors and Tregs,43 and CMAF in TFH and TH17 cells.44 This complicates the interpretation of these TMA-IHC findings in isolation. However, combining functional and flow phenotype data from the SCS work, a predominance of TBET and absence of CMAF/GATA3 is highly suggestive of a TH1-polarized immune infiltrate in CHL with a surprisingly absent TH2 response. Overall, therefore, we find little evidence that TH2 bias can explain the failure of antitumor immunity in CHL, although we acknowledge that cells expressing a particular combination of cytokines, CCRs, and polarizing transcription factors in vitro may behave differently in the complex multicellular environment in vivo.
The evidence for a TH2 bias in CHL was based largely on early immunophenotyping data,45 which showed that the majority of tumor-infiltrating cells are CD45RO+, CD45RA−, and CD45RBlo. Although these results are consistent with our own immunophenotyping data, they have been previously interpreted as suggesting excess TH2-biased cells. This interpretation was in the context of a more limited understanding of CD45 isoforms.46 It is now understood that CD45 isoform expression in T cells discriminates naive from antigen-exposed cells and proliferative from secretory/effector cells and may be an indicator of overall functional plasticity rather than TH1/TH2 polarization (for a review, see Penninger et al47).
A TH1-biased infiltrate may be hypothesized to be accompanied by a more extensive cytotoxic T-cell infiltrate, and indeed we found a positive association between numbers of CD8+ T cells and expression of TBET. However, our previous publication34 reported no association between numbers of CD8+ cells and clinical outcome, although perhaps unexpectedly a number of previous groups have shown an adverse impact of microenvironment expression of markers of cytotoxic T-cell function, Granzyme B, and TIA1.20,48 Our study used the material available to focus on markers of CD4+ function and as such did not investigate these markers.
PD-L1 overexpression by HRS cells is well described,11,49,50 but there is a paucity of evidence for expression of its ligand PD1 in the CHL microenvironment.13,14 and we confirm strikingly low expression levels. This suggests that PD1/PD-L1 immunosuppressive interactions cannot readily explain the failure of the immune response to eradicate tumor. PD1 appears to be important in the small minority of cases in which it is expressed and in whom it is associated with adverse prognosis, confirming a previous study.16 Based on our previous study of the significant infiltrate of FOXP3+ cells in CHL, we investigated expression of CTLA4, an immunosuppressive molecule overexpressed by FOXP3+ Tregs as well as by activated effector T cells, and found it expressed to a greater extent in CHL than in RLN TH cells, although in the SCS, median FOXP3 expression was similar in CHL and RLN-TH cells.
CTLA4 is also nonspecifically upregulated in activated nonregulatory T cells. We find extensive evidence for T-cell activation with overexpression of markers of central memory and upregulation of activation-induced molecules, including ligand/receptor pairs for surface molecules expressed by the HRS cell and a capacity for cytokine-induced proliferation of the TH component of CHL to a similar extent as that seen in reactive nodes.
Rosetting of the HRS cell by T cells is frequently reported in studies of the histological architecture of CHL. We found no spatial relationship between expression of TBET, GATA3, or CMAF in this study, with markers being relatively evenly distributed throughout the lymphoid microenvironment. We acknowledge that a limitation of using SCS is that no comment can be made on spatial relationships between T cells expressing activation molecules and HRS cells expressing their receptor/ligand reciprocal, and such an investigation in TMA would be an important component of future work.
Despite the heterogeneity of expression levels of markers between samples, reflecting disease heterogeneity, our data do suggest a characteristic immunophenotype of the CHL-TH cell, namely proinflammatory, cytokine-secretory, rich in CM cells, lacking TH2-associated CCR3 or IL-4/-13 and senescence markers PD1, CD57, or TEMRA, and overexpressing TH1-associated CXCR3/CCR5 and activation-induced CD153/CD30-L, CD134/OX40, CD278/ICOS, CD152/CTLA4, CD95, and CD45RO. This appears to be a pathognomonic “activated” signature of the CHL microenvironment, perhaps suitable for diagnostic application in histologically ambiguous cases.
The evidence suggests that the malignant tumor is capable of sequestering substantial numbers of activated, functional T cells, providing one explanation for the profound systemic immune defect encountered in advanced disease. Overexpression of reciprocal receptor/ligand pairs for HRS cell-expressed molecules capable of transducing survival signals suggests a number of potentially important therapeutic targets, all of which should continue to be explored. Overall, we find little to support the hypothesis that the TH infiltrate is TH2 biased or exhausted/senescent, but rather we find evidence for an activated, proliferative, and proinflammatory cytokine-secretory phenotype.
Acknowledgments
This work was supported by The Baker Foundation, Cancer Research UK and by a National Cancer Institute Programme Grant (PO1 C81538 to J.G.G.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: P.G. designed and performed the research, performed statistical analysis and results interpretation, and wrote the paper; A.C. and A.O. constructed the TMA and optimized the IHC reagents; S.I. collected and processed the SCS for flow; J.M. and A.W. managed patient database and performed statistical analysis; A.L. provided expert histopathological review; M.C. provided expert histopathological review and supervised the project; and J.G.G. designed the research, supervised the project and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul Greaves, Centre for Haemato-oncology, Bart’s Cancer Institute, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, Charterhouse Square, London EC1M 5BQ, United Kingdom; e-mail: greaves.paul@gmail.com.
References
- 1.Steidl C, Connors JM, Gascoyne RD. Molecular pathogenesis of Hodgkin’s lymphoma: increasing evidence of the importance of the microenvironment. J Clin Oncol. 2011;29(14):1812–1826. doi: 10.1200/JCO.2010.32.8401. [DOI] [PubMed] [Google Scholar]
- 2.Van Pel A, Vessière F, Boon T. Protection against two spontaneous mouse leukemias conferred by immunogenic variants obtained by mutagenesis. J Exp Med. 1983;157(6):1992–2001. doi: 10.1084/jem.157.6.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nastala CL, Edington HD, McKinney TG, et al. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol. 1994;153(4):1697–1706. [PubMed] [Google Scholar]
- 4.Lowes MA, Bishop GA, Crotty K, Barnetson RS, Halliday GM. T helper 1 cytokine mRNA is increased in spontaneously regressing primary melanomas. J Invest Dermatol. 1997;108(6):914–919. doi: 10.1111/1523-1747.ep12292705. [DOI] [PubMed] [Google Scholar]
- 5.Pellegrini P, Berghella AM, Del Beato T, Cicia S, Adorno D, Casciani CU. Disregulation in TH1 and TH2 subsets of CD4+ T cells in peripheral blood of colorectal cancer patients and involvement in cancer establishment and progression. Cancer Immunol Immunother. 1996;42(1):1–8. doi: 10.1007/s002620050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kacha AK, Fallarino F, Markiewicz MA, Gajewski TF. Cutting edge: spontaneous rejection of poorly immunogenic P1.HTR tumors by Stat6-deficient mice. J Immunol. 2000;165(11):6024–6028. doi: 10.4049/jimmunol.165.11.6024. [DOI] [PubMed] [Google Scholar]
- 7.Sartoris S, Cavallero P, Pegoraro L, Vergnano F, Fazio M. The absence of lymphocyte response in vitro to tuberculin challenge in Hodgkin’s disease. Panminerva Med. 1965;7(10):370–372. [PubMed] [Google Scholar]
- 8.Han T, Sokal JE. Lymphocyte response to phytohemagglutinin in Hodgkin’s disease. Am J Med. 1970;48(6):728–734. doi: 10.1016/s0002-9343(70)80008-0. [DOI] [PubMed] [Google Scholar]
- 9.Marshall NA, Christie LE, Munro LR, et al. Immunosuppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood. 2004;103(5):1755–1762. doi: 10.1182/blood-2003-07-2594. [DOI] [PubMed] [Google Scholar]
- 10.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 11.Steidl C, Telenius A, Shah SP, et al. Genome-wide copy number analysis of Hodgkin Reed-Sternberg cells identifies recurrent imbalances with correlations to treatment outcome. Blood. 2010;116(3):418–427. doi: 10.1182/blood-2009-12-257345. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto R, Nishikori M, Kitawaki T, et al. PD-1-PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood. 2008;111(6):3220–3224. doi: 10.1182/blood-2007-05-085159. [DOI] [PubMed] [Google Scholar]
- 13.Dorfman DM, Brown JA, Shahsafaei A, Freeman GJ. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am J Surg Pathol. 2006;30(7):802–810. doi: 10.1097/01.pas.0000209855.28282.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nam-Cha SH, Roncador G, Sanchez-Verde L, et al. PD-1, a follicular T-cell marker useful for recognizing nodular lymphocyte-predominant Hodgkin lymphoma. Am J Surg Pathol. 2008;32(8):1252–1257. doi: 10.1097/PAS.0b013e318165b0d6. [DOI] [PubMed] [Google Scholar]
- 15.Carbone A, Gloghini A, Cabras A, Elia G. Differentiating germinal center-derived lymphomas through their cellular microenvironment. Am J Hematol. 2009;84(7):435–438. doi: 10.1002/ajh.21434. [DOI] [PubMed] [Google Scholar]
- 16.Muenst S, Hoeller S, Dirnhofer S, Tzankov A. Increased programmed death-1+ tumor-infiltrating lymphocytes in classical Hodgkin lymphoma substantiate reduced overall survival. Hum Pathol. 2009;40(12):1715–1722. doi: 10.1016/j.humpath.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 17.Andersson E, Dahlenborg K, Ohlin M, Borrebaeck CA, Carlsson R. Immunoglobulin production induced by CD57+ GC-derived helper T cells in vitro requires addition of exogenous IL-2. Cell Immunol. 1996;169(2):166–173. doi: 10.1006/cimm.1996.0107. [DOI] [PubMed] [Google Scholar]
- 18.Zhu JF, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112(5):1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atayar C, van den Berg A, Blokzijl T, et al. Hodgkin’s lymphoma associated T-cells exhibit a transcription factor profile consistent with distinct lymphoid compartments. J Clin Pathol. 2007;60(10):1092–1097. doi: 10.1136/jcp.2006.044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schreck S, Friebel D, Buettner M, et al. Prognostic impact of tumour-infiltrating Th2 and regulatory T cells in classical Hodgkin lymphoma. Hematol Oncol. 2009;27(1):31–39. doi: 10.1002/hon.878. [DOI] [PubMed] [Google Scholar]
- 21.Gruss HJ, Pinto A, Duyster J, Poppema S, Herrmann F. Hodgkin’s disease: a tumor with disturbed immunological pathways. Immunol Today. 1997;18(4):156–163. doi: 10.1016/s0167-5699(97)84661-0. [DOI] [PubMed] [Google Scholar]
- 22.Frisan T, Sjöberg J, Dolcetti R, et al. Local suppression of Epstein-Barr virus (EBV)-specific cytotoxicity in biopsies of EBV-positive Hodgkin’s disease. Blood. 1995;86(4):1493–1501. [PubMed] [Google Scholar]
- 23.Serrano D, Ghiotto F, Roncella S, et al. The patterns of IL2, IFN-gamma, IL4 and IL5 gene expression in Hodgkin’s disease and reactive lymph nodes are similar. Haematologica. 1997;82(5):542–549. [PubMed] [Google Scholar]
- 24.Poppema S, Potters M, Visser L, van den Berg AM. Immune escape mechanisms in Hodgkin’s disease. Ann Oncol. 1998;9(Suppl 5):S21–S24. doi: 10.1093/annonc/9.suppl_5.s21. [DOI] [PubMed] [Google Scholar]
- 25.Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277(5334):2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 26.Bonecchi R, Bianchi G, Bordignon PP, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187(1):129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hedvat CV, Jaffe ES, Qin J, et al. Macrophage-derived chemokine expression in classical Hodgkin’s lymphoma: application of tissue microarrays. Mod Pathol. 2001;14(12):1270–1276. doi: 10.1038/modpathol.3880473. [DOI] [PubMed] [Google Scholar]
- 28.Maggio EM, Van Den Berg A, Visser L, et al. Common and differential chemokine expression patterns in rs cells of NLP, EBV positive and negative classical Hodgkin lymphomas. Int J Cancer. 2002;99(5):665–672. doi: 10.1002/ijc.10399. [DOI] [PubMed] [Google Scholar]
- 29.Ohshima K, Tutiya T, Yamaguchi T, et al. Infiltration of Th1 and Th2 lymphocytes around Hodgkin and Reed-Sternberg (H&RS) cells in Hodgkin disease: relation with expression of CXC and CC chemokines on H&RS cells. Int J Cancer. 2002;98(4):567–572. doi: 10.1002/ijc.10218. [DOI] [PubMed] [Google Scholar]
- 30.Buri C, Körner M, Schärli P, et al. CC chemokines and the receptors CCR3 and CCR5 are differentially expressed in the nonneoplastic leukocytic infiltrates of Hodgkin disease. Blood. 2001;97(6):1543–1548. doi: 10.1182/blood.v97.6.1543. [DOI] [PubMed] [Google Scholar]
- 31.Küppers R. The biology of Hodgkin’s lymphoma. Nat Rev Cancer. 2009;9(1):15–27. doi: 10.1038/nrc2542. [DOI] [PubMed] [Google Scholar]
- 32.Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1(5):433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 33.Belldegrun A, Muul LM, Rosenberg SA. Interleukin 2 expanded tumor-infiltrating lymphocytes in human renal cell cancer: isolation, characterization, and antitumor activity. Cancer Res. 1988;48(1):206–214. [PubMed] [Google Scholar]
- 34.Greaves P, Clear A, Coutinho R, et al. Expression of FOXP3, CD68, and CD20 at diagnosis in the microenvironment of classical Hodgkin lymphoma is predictive of outcome. J Clin Oncol. 2013;31(2):256–262. doi: 10.1200/JCO.2011.39.9881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 36.Concato J, Feinstein AR, Holford TR. The risk of determining risk with multivariable models. Ann Intern Med. 1993;118(3):201–210. doi: 10.7326/0003-4819-118-3-199302010-00009. [DOI] [PubMed] [Google Scholar]
- 37.Leonard WJ, Spolski R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol. 2005;5(9):688–698. doi: 10.1038/nri1688. [DOI] [PubMed] [Google Scholar]
- 38.Scheeren FA, Diehl SA, Smit LA, et al. IL-21 is expressed in Hodgkin lymphoma and activates STAT5: evidence that activated STAT5 is required for Hodgkin lymphomagenesis. Blood. 2008;111(9):4706–4715. doi: 10.1182/blood-2007-08-105643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skinnider BF, Elia AJ, Gascoyne RD, et al. Interleukin 13 and interleukin 13 receptor are frequently expressed by Hodgkin and Reed-Sternberg cells of Hodgkin lymphoma. Blood. 2001;97(1):250–255. doi: 10.1182/blood.v97.1.250. [DOI] [PubMed] [Google Scholar]
- 40.Barros MH, Vera-Lozada G, Soares FA, Niedobitek G, Hassan R. Tumor microenvironment composition in pediatric classical Hodgkin lymphoma is modulated by age and Epstein-Barr virus infection. Int J Cancer. 2012;131(5):1142–1152. doi: 10.1002/ijc.27314. [DOI] [PubMed] [Google Scholar]
- 41.Chetaille B, Bertucci F, Finetti P, et al. Molecular profiling of classical Hodgkin lymphoma tissues uncovers variations in the tumor microenvironment and correlations with EBV infection and outcome. Blood. 2009;113(12):2765–3775. doi: 10.1182/blood-2008-07-168096. [DOI] [PubMed] [Google Scholar]
- 42.Lazarevic V, Glimcher LH. T-bet in disease. Nat Immunol. 2011;12(7):597–606. doi: 10.1038/ni.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Su MA, Wan YY. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity. 2011;35(3):337–348. doi: 10.1016/j.immuni.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bauquet AT, Jin H, Paterson AM, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009;10(2):167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poppema S. The nature of the lymphocytes surrounding Reed-Sternberg cells in nodular lymphocyte predominance and in other types of Hodgkin’s disease. Am J Pathol. 1989;135(2):351–357. [PMC free article] [PubMed] [Google Scholar]
- 46.Morimoto C, Letvin NL, Distaso JA, Aldrich WR, Schlossman SF. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985;134(3):1508–1515. [PubMed] [Google Scholar]
- 47.Penninger JM, Irie-Sasaki J, Sasaki T, Oliveira-dos-Santos AJ. CD45: new jobs for an old acquaintance. Nat Immunol. 2001;2(5):389–396. doi: 10.1038/87687. [DOI] [PubMed] [Google Scholar]
- 48.Alvaro T, Lejeune M, Salvadó MT, et al. Outcome in Hodgkin’s lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res. 2005;11(4):1467–1473. doi: 10.1158/1078-0432.CCR-04-1869. [DOI] [PubMed] [Google Scholar]
- 49.Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116(17):3268–3277. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steidl C, Shah SP, Woolcock BW, et al. MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nature. 2011;471(7338):377–381. doi: 10.1038/nature09754. [DOI] [PMC free article] [PubMed] [Google Scholar]