Abstract
A purified recombinant protein from Eimeria acervulina (3-1E) was used to vaccinate chickens in ovo against coccidiosis both alone and in combination with expression plasmids encoding the interleukin 1 (IL-1), IL-2, IL-6, IL-8, IL-15, IL-16, IL-17, IL-18, or gamma interferon (IFN-γ) gene. When used alone, vaccination with 100 or 500 μg of 3-1E resulted in significantly decreased oocyst shedding compared with that in nonvaccinated chickens. Simultaneous vaccination of the 3-1E protein with the IL-1, -15, -16, or -17 gene induced higher serum antibody responses than 3-1E alone. To evaluate protective intestinal immunity, vaccinated birds were challenged with live E. acervulina oocysts 14 days posthatch, and fecal-oocyst shedding and body weight gain were determined as parameters of coccidiosis. Chickens vaccinated with 3-1E protein showed significantly lower oocyst shedding and normal body weight gain than nonvaccinated and infected controls. Simultaneous immunization with 3-1E and the IL-2, -15, -17, or -18 or IFN-γ gene further reduced oocyst shedding compared with that achieved with 3-1E alone. These results provide the first evidence that in ovo vaccination with the recombinant 3-1E Eimeria protein induces protective intestinal immunity against coccidiosis, and this effect was enhanced by coadministration of genes encoding immunity-related cytokines.
Coccidiosis is an economically important disease that seriously impairs the feed utilization and growth of chickens due to intestinal infection with the protozoan Eimeria (1, 16, 17). Anticoccidial drugs, as well as live and attenuated parasite vaccines, are available to control field outbreaks of infection (5, 47). However, increasing regulatory restrictions on antibiotic use in poultry production and the limited efficacies of whole-parasite vaccines against field strains of Eimeria have spawned an interest in alternative strategies for disease control (16, 43). In this regard, recombinant subunit vaccines have recently shown promise in inducing protective immunity against parasite infection (17). Over the past 10 years, Eimeria genes encoding protective antigens have been cloned, and their gene products have been characterized. These include parasite surface proteins (15), as well as internal proteins associated with subcellular organelles, such as the rhoptries (40), microneme (41), and refractile bodies (42). Although many of the reported Eimeria recombinant proteins elicit some aspects of host immunity in vaccinated recipients (16, 43), the natures of these protective antigens need to be better characterized, particularly with respect to their roles in parasite growth and development and the infectious process (45, 46).
Since Eimeria spp. are primarily intestinal parasites, the route of delivery of recombinant vaccines is critical in establishing effective stimulation of local immunity. Initially, recombinant vaccines were given by intramuscular or subcutaneous immunization using purified proteins or DNA expression plasmids carrying the gene of interest (9, 12, 13). More recently, alternative routes of vaccination have been used successfully, including intranasal, intraocular, and in ovo immunization. Vaccination of eggs represents a promising avenue of delivery, and over the past 2 decades it has been shown to be a safe, effective, and convenient method of administering many different agents, including drugs, nutrients, immunogens, and genes (11, 31). In ovo administration of protein-based, as well as live or attenuated, vaccines has been experimentally demonstrated to be an effective method of immunization against many different avian diseases and is routinely performed in the commercial broiler industry for selected pathogens (6, 21, 30, 35-38, 44). In the case of coccidiosis, in ovo vaccination using live Eimeria parasites (45, 46) has shown promising results for disease management in production settings, although a drawback to any immunization protocol using viable coccidia is posthatch fecal shedding of infectious oocysts.
Recent studies have demonstrated enhanced efficiency of recombinant vaccines in producing protective immunity by simultaneous injection of cytokines (2, 14, 15, 18-20, 22, 25). Reddy et al. (29) reported improved virus-specific immune responses in cows given a live bovine herpesvirus 1 vaccine plus interleukin 1β (IL-1β) compared with the vaccine alone. Recombinant IL-5 and IL-6 markedly increased IgA titers to coexpressed heterologous antigens in mice (27). Pasquini et al. (26) reported that IL-2, IL-4, IL-12, and gamma interferon (IFN-γ) amplified immune responses to genetic vaccines. Unlike classical adjuvant, such as mineral oils and Freund's adjuvant, cytokine adjuvants initiate antigen-presenting-cell polarization and costimulatory molecule expression (4). In chickens, subcutaneous injection of IL-2 enhanced T-lymphocyte and NK cell numbers and functions, while injection of IFN-γ reduced oocyst production and improved body weight gain following challenge with live Eimeria (14, 15, 18). Injection of expression plasmids encoding IFN-α or lymphotactin enhanced body weight gain when given simultaneously with a gene encoding a recombinant subunit vaccine (3-1E), an immunogenic 20-kDa protein highly conserved among various life cycle stages and species of Eimeria (15, 39). More recently, it was found that the 3-1E protein is synthesized in all sexual stages of Eimeria tenella, stimulates cell-mediated immunity, and contains a putative conserved domain for the actin-regulatory protein profilin (unpublished observations). The protein encoded by 3-1E in combination with another cytokine (IL-1β, IL-8, IL-15, IFN-γ, or transforming growth factor β4) significantly decreased fecal-oocyst shedding (22). The present study was carried out to investigate the induction of protective intestinal immunity against Eimeria acervulina using in ovo injection of the recombinant 3-1E protein both alone and in conjunction with genes encoding cytokine adjuvants.
MATERIALS AND METHODS
Chickens and in ovo immunization.
Specific-pathogen-free embryonated eggs of inbred white Leghorn SC chickens (HyLine International, Dallas Center, Iowa) were hatched at the Animal and Natural Resources Institute (Beltsville, Md.), and the chicks were provided with commercial broiler ration and water ad libitum. For in ovo immunization, eggs were incubated for 18 days, candled to select fertile eggs, and injected with the 3-1E recombinant protein. Briefly, 3-1E in 100 μl of sterile phosphate-buffered saline (PBS), pH 7.4, was injected into the amniotic sac by using the entire length of a 2.85-cm-long 18-gauge needle (Intelliject; Avitech, Hebron, Md.) according to the manufacturer's instructions (38). All experiments were performed according to guidelines established by the Beltsville Agriculture Research Center Small Animal Care Committee.
Parasites.
The wild-type strain of E. acervulina, originally developed at the Livestock and Poultry Science Institute (Beltsville, Md.), was used for oral inoculations. Oocysts were cleaned by flotation on 5.25% sodium hypochloride, washed three times with PBS, and enumerated by hemocytometry.
Expression and purification of the 3-1E recombinant protein.
3-1E was expressed and purified as described previously (15, 39). Briefly, the 3-1E gene cloned in the pMAL plasmid with an NH2-terminal maltose-binding protein tag was expressed in competent Escherichia coli DH5α in 1× TY broth (20 g of tryptone/liter, 10 g of yeast extract/liter, 10 g of NaCl/liter) containing 100 μg of ampicillin/ml, and the bacteria were grown to an optical density at 600 nm (OD600) of 0.5, induced with 1.0 mM isopropyl-β-d-thiogalactopyranoside for 3 h at 37°C, collected by centrifugation, and disrupted by sonication on ice (Misonix, Farmingdale, N.Y.). The 3-1E protein was isolated on an amylose affinity column (New England Biolabs, Beverly, Mass.) according to the manufacturer's instructions, digested with Factor Xa to release 3-1E, and repassed through the amylose column to remove maltose-binding protein. Final purity was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western hybridization.
Chicken cytokine genes.
cDNAs encoding IL-1, IL-2, IL-6, IL-8, IL-15, IL-16, IL-17, and IFN-γ have been described (15, 22-24). An expression plasmid encoding IL-18 (33) was generously provided by Peter Staeheli (University of Freiberg, Freiberg, Germany). The plasmids were transformed into E. coli DH5α, grown for 14 to 16 h, and purified using a commercial kit (QIAGEN, Valencia, Calif.) according to the manufacturer's instructions. DNA was quantified spectrophotometrically.
3-1E antibody enzyme-linked immunosorbent assay.
Flat-bottom, 96-well microtiter plates (Costar, Boston, Mass.) were coated with 100 μl of purified 3-1E protein (10 μg/ml) in 0.1 M sodium carbonate buffer, pH 9.6, at 4°C overnight and washed two times with PBS, pH 7.2, containing 0.05% Tween 20 (PBST). The wells were blocked with 100 μl of PBS-1% bovine serum albumin (Sigma) for 1 h at room temperature, followed by 100 μl of serum for 2 h at room temperature. The wells were washed five times with PBST and incubated for 30 min at room temperature with 100 μl of horseradish peroxidase-conjugated anti-chicken immunoglobulin G (IgG) (Sigma) diluted 1:4,000 in PBS-1% bovine serum albumin. The wells were washed five times with PBST and developed with 100 μl of 0.01% (wt/vol) tetramethylbenzidine (Sigma) in 0.05 M phosphate-citrate buffer, pH 5.0, for 10 min followed by 50 μl of 2N H2SO4, and the OD450 was determined with a microplate spectrophotometer.
Statistical analysis.
Mean values for body weights and antibody titers were compared by the Yuker-Kramer multiple-comparison test. Mean values for fecal-oocyst shedding were compared by the Dunnett multiple-comparison test. Differences between means were considered significant at a P value of <0.05.
Experimental design. (i) Experiment 1.
To determine the optimal dose of 3-1E protein required to elicit a serum antibody response, 75 eggs were distributed into five groups (15/group) and vaccinated with 100 μl of PBS or 25, 50, 100, or 200 μg of purified 3-1E.
(ii) Experiment 2.
To determine the optimal dose of 3-1E protein required to elicit protective immunity, 90 eggs were distributed into six groups (15/group) and vaccinated with 100 μl of PBS (groups 1 and 2) or 25, 50, 100, or 500 μg of purified 3-1E (groups 3 to 7). At 14 days posthatch, the chickens were not infected (group 1) or were orally infected with 10,000 E. acervulina oocysts (groups 2 to 6). Serum samples were collected 10 days after parasite infection for 3-1E-reactive antibody titers (five chickens/group). Fecal oocyst shedding was determined daily at 5 to 10 days postinfection (five chickens/group), and body weights were measured at 0 and 6 days postinfection (five chickens/group).
(iii) Experiment 3.
To investigate the effects of chicken cytokines on protective immunity following 3-1E vaccination, 180 eggs were distributed into 12 groups (15/group) and vaccinated with 100 μl of PBS (groups 1 and 2), 100 μg of 3-1E plus 5.0 μg of the pcDNA empty vector (group 3), or 3-1E plus 5.0 μg of purified plasmid DNA containing IL-1, -2, -6, -8, -15, -16, -17, or -18 or IFN-γ cDNA (groups 4 to 12). At 14 days posthatch, the chickens were not infected (group 1) or were orally infected with 10,000 E. acervulina oocysts (groups 2 to 12). E. acervulina serum IgG antibody titers, body weights, and fecal-oocyst shedding were determined as described above.
RESULTS
Effects of in ovo vaccination with 3-1 protein on hatching and survival. Initially, we determined hatchability and survival at 24 days posthatch following in ovo vaccination at 18 days of embryonation with recombinant 3-1E protein with or without cytokine genes. In the three experimental protocols, in ovo-vaccinated chickens showed 92 to 97% hatching and 90 to 94% survival (Table 1). These percentages were not statistically different from those for eggs given PBS alone, where hatching and survival were 93 to 100% and 93 to 97%, respectively.
TABLE 1.
Effects of in ovo vaccination with 3-1E protein on hatchability and posthatch survival
| Expt | Vaccination | No. of eggs/group | % Hatchinga | % Survivalb |
|---|---|---|---|---|
| 1 | PBS | 15 | 93 | 93 |
| 3-1E | 60 | 92 | 90 | |
| 2 | PBS | 30 | 100 | 97 |
| 3-1E | 60 | 97 | 93 | |
| 3 | PBS | 30 | 97 | 93 |
| 3-1E | 150 | 96 | 94 |
% Hatching = (number of hatched chickens/number of vaccinated eggs) × 100%.
% Survival = (number of chickens alive 24 days posthatch/number of vaccinated eggs) × 100%.
Effects of in ovo vaccination with 3-1E protein on E. acervulina infection.
To investigate the development of protective immunity following in ovo immunization with purified 3-1E protein, eggs were vaccinated with PBS or 25, 50, 100, or 500 μg of 3-1E, and the chickens were orally infected with 10,000 sporulated oocysts of E. acervulina at 14 days posthatch. Fecal oocyst shedding between 5 and 10 days postinfection and body weights at 0 and 6 days postinfection were assessed as parameters of protective immunity. Serum IgG antibodies specific for 3-1E protein were detected at 1 week posthatch in groups immunized with 25 and 50 μg of immunogen, while those following 100- and 200-μg immunizations were greatest at 2 and 3 weeks posthatch (data not shown).
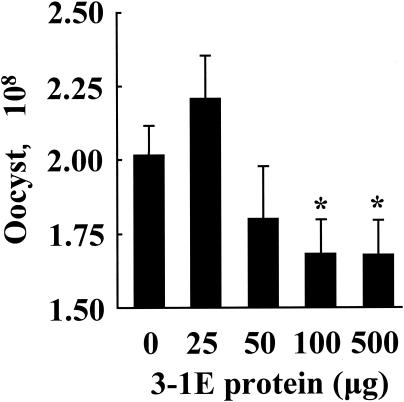
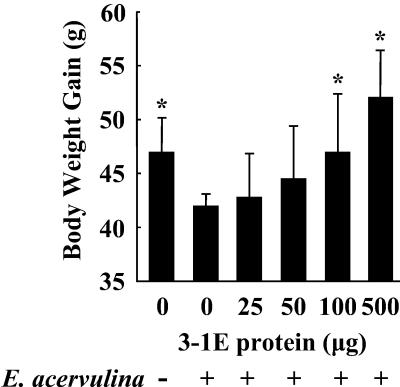
As shown in Fig. 1, vaccination with 100 or 500 μg of 3-1E resulted in significantly decreased oocyst shedding compared with nonvaccinated (PBS-vaccinated) chickens. As shown in Fig. 2, 3-1E vaccination also reversed body weight loss associated with E. acervulina infection. Thus, nonvaccinated and parasite-infected chickens showed an ∼15% decrease in body weight gain during the 6-day observation period compared with noninfected controls (P < 0.05). Vaccination with 100 or 500 μg of 3-1E reversed this effect so that body weight gains at these inocula were significantly greater than those in the nonvaccinated and infected group (P < 0.05).
FIG. 1.
Fecal-oocyst shedding following vaccination with 3-1E protein. Chickens were vaccinated in ovo with PBS (0) or 25, 50, 100, or 500 μg of 3-1E and orally infected with 10,000 oocysts of E. acervulina at 14 days posthatch, and cumulative fecal-oocyst numbers were determined between 5 and 10 days postinfection. Each bar represents the mean plus standard error of the mean (n = 5). The asterisks indicate significantly decreased oocyst numbers compared with the PBS control.
FIG. 2.
Body weight gain following vaccination with 3-1E protein. Chickens were vaccinated in ovo with PBS (0) or 25, 50, 100, or 500 μg of 3-1E and either not infected (−) or orally infected with 10,000 oocysts of E. acervulina (+) at 14 days posthatch. Body weights were determined at 0 and 6 days postinfection. Each bar represents the mean plus standard error of the mean (n = 5). The asterisks indicate significantly increased weight gains compared with the nonvaccinated and infected control.
Effects of in ovo covaccination with 3-1E protein and cytokine genes.
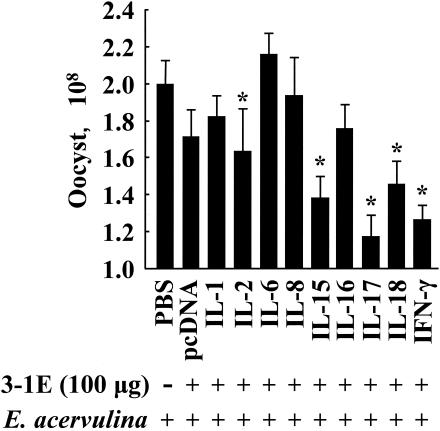
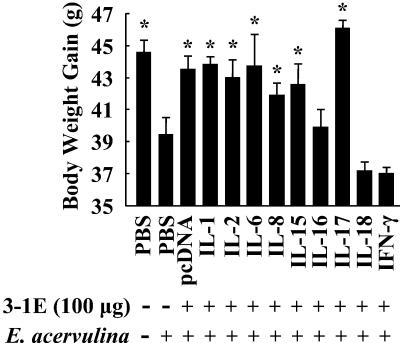
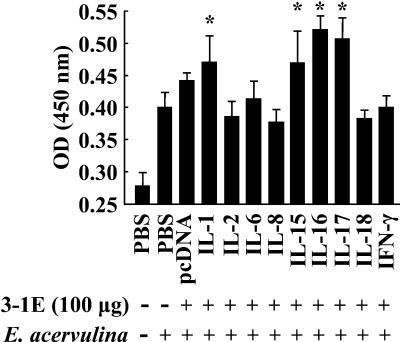
To investigate the effects of covaccination with 3-1E and cytokine genes, eggs were vaccinated with PBS or 100 μg of 3-1E in combination with 5.0 μg of plasmid DNA from the pcDNA empty vector or DNA encoding IL-1, -2, -6, -8, -15, -16, -17, and -18 and IFN-γ. At 14 days posthatch, the chickens were challenged orally with E. acervulina, and fecal-oocyst shedding and body weight gain were assessed as described above. As shown in Fig. 3, oocyst shedding following vaccination with 3-1E plus the IL-2, -15, -17, or -18 or IFN-γ gene was significantly reduced compared with 3-1E plus the pcDNA empty vector (P < 0.05 for IL-2; P < 0.001 for IL-15, -17, or -18 or IFN-γ). In contrast, 3-1E plus IL-6 or -8 increased oocyst shedding to the level seen in the nonvaccinated and infected controls. As shown in Fig. 4, covaccination with 3-1E protein plus the IL-1, -2, -6, -8, -15, or -17 gene resulted in body weight gains equal to those produced by immunization with 3-1E plus empty vector alone. However, coadministration of the IL-16, IL-18, or IFN-γ gene had a negative effect on body weight gain induced by 3-1E vaccination: 3-1E plus these cytokines resulted in weight gain equal to that of the nonvaccinated and infected group. Finally, we also measured 3-1E-reactive serum antibody titers in 3-1E plus cytokine gene covaccinees at 10 days postinfection. As shown in Fig. 5, animals receiving 3-1E in combination with the the IL-1, -15, -16, or -17 gene exhibited significantly greater serum IgG antibody levels than with 3-1E plus vector alone (P < 0.05).
FIG. 3.
Fecal-oocyst shedding following covaccination with 3-1E protein plus cytokine genes. Chickens were vaccinated in ovo with PBS (−) or 100 μg of 3-1E protein (+) plus 5.0 μg of plasmid DNAs from the pcDNA empty vector or encoding the indicated cytokine genes and orally infected with 10,000 oocysts of E. acervulina at 14 days posthatch, and fecal-oocyst numbers were determined between 5 and 10 days postinfection. Each bar represents the mean plus standard error of the mean (n = 5). The asterisks indicate significantly decreased oocyst numbers compared with the 3-1E plus vector only group.
FIG. 4.
Body weight gain following covaccination with 3-1E protein plus cytokine genes. Chickens were vaccinated in ovo with PBS (−) or 100 μg of 3-1E (+) plus 5.0 μg of plasmid DNAs from the pcDNA empty vector or encoding the indicated cytokine genes. At 14 days posthatch, the chickens were either not infected (−) or orally infected with 10,000 oocysts of E. acervulina (+), and body weights were determined at 0 and 6 days postinfection. Each bar represents the mean plus standard error of the mean (n = 5). The asterisks indicate significantly increased weight gains compared with the nonvaccinated and infected control (P < 0.05).
FIG. 5.
Serum IgG antibody titers following covaccination with 3-1E protein plus cytokine genes. Chickens were vaccinated in ovo with PBS (−) or 100 μg of 3-1E (+) plus 5.0 μg of plasmid DNAs from the pcDNA empty vector or encoding the indicated cytokine genes. At 14 days posthatch, the chickens were either not infected (−) or orally infected with 10,000 oocysts of E. acervulina (+), and anti-3-1E serum antibody levels were determined by enzyme-linked immunosorbent assay. Each bar represents the mean plus standard error of the mean (n = 5) of triplicate determinations. The asterisks indicate significantly increased OD450 values compared with the 3-1E plus vector only group (P < 0.05).
DISCUSSION
The results obtained in this study demonstrate that in ovo vaccination with the 3-1E protein generates immunogen-reactive serum IgG antibodies, and the humoral response was enhanced by covaccination with 3-1E plus the IL-1, -16, -17, or -18 gene. Furthermore, vaccination with 3-1E augmented protective immunity against E. acervulina infection as measured by reduced fecal-oocyst shedding and increased body weight gain compared with nonvaccinated controls. Covaccination with 3-1E plus the IL-2, -15, -17, or -18 or IFN-γ gene led to further reduction of oocyst shedding beyond that induced by 3-1E alone. However, covaccination with 3-1E plus IL-1, -2, -6, -8, -15, or -17 led to body weight gains equal to those achieved by 3-1E alone. Furthermore, 3-1E plus IL-16 or -18 or IFN-γ negated the increase in weight gain produced by 3-1E alone, so that the gains in these groups were equal to those in the nonvaccinated and infected controls.
In ovo-vaccinated chickens develop humoral and cellular immunities that may or may not contribute to posthatch protection against infection, depending on the nature of the immunogen. Chicken embryos were initially shown to synthesize serum gamma globulin during the third week of incubation (28). Although compared with the adult, young chicks exhibit diminished antibody responses due to immature T lymphocytes, the birds are nonetheless capable of developing partial immunity following vaccination of the embryo (3). Indeed, the pioneering studies by Sharma and coworkers (35-38) demonstrated that in ovo vaccination was an effective method to induce immunity to a variety of economically important avian viruses. With respect to coccidiosis, vaccination of broiler chicken embryos with Eimeria oocysts induced partial immunity against a subsequent challenge with the live parasite (45, 46). In contrast, Hornok et al. (7) showed that in ovo vaccination using a Cryptosporidium baileyi oocyst extract did not induce a protective immune response but rather impaired immunity and delayed clearance of cryptosporidia from the chickens.
Due to the severe economic cost associated with coccidiosis in commercial settings, much research has focused on vaccination protocols as potential methods of disease control. Vaccination offers a promising means of preventing coccidiosis, since Eimeria infection in natural settings induces strong protective immunity (10). Currently available immunological control strategies consist of subacute infection with virulent or live and attenuated parasites. For reasons of safety and cost, however, these methods are undesirable, and many attempts have been made to identify immunogenic epitopes of Eimeria for the development of subunit vaccines. Toward this end, our laboratory originally cloned and characterized the 3-1E gene and purified its corresponding gene product as a potential subunit vaccine candidate (15). The 3-1E cDNA was isolated from E. acervulina merozoites and contained a 1,086-bp insertion predicted to encode an open reading frame of 170 amino acids with a molecular weight of 18,523. By immunofluorescence staining, a monoclonal antibody produced against the recombinant 3-1E protein reacted with sporozoites and merozoites of E. acervulina, E. tenella, and Eimeria maxima, suggesting that 3-1E expression was common to different life cycle stages and parasite species. It was subsequently shown that intramuscular or subcutaneous vaccination with the 3-1E protein or cDNA significantly reduced fecal-oocyst shedding and enhanced humoral and cell-mediated immune responses (39). In the present study, we have confirmed our original observations and extended them to demonstrate the efficacy of in ovo 3-1E vaccination and enhanced protective immunity when used in combination with cytokine adjuvants. In total, these results indicate that the recombinant 3-1E protein is a promising vaccine candidate for controlling coccidiosis in the field.
The use of cytokines as adjuvants has become a powerful method to enhance immunity elicited by recombinant DNA or protein vaccines. Because of the lack of sequence homology between avian and mammalian cytokines, molecular cloning of chicken cytokine genes, in many cases, has not achieved the same level of sophistication as in mammals. In addition to the cytokines utilized in this study, chicken homologues of IFN-α, lymphotactin, and transforming growth factor β have been cloned (8, 32, 34) and have shown promise as immunologic costimulators (22). One problem with the in vivo use of cytokines is their rapid degradation and clearance (20). This problem is overcome by administering them in DNA form, which may provide a more practical method for field vaccination. In avian coccidiosis, administration of a recombinant IFN-γ gene by itself decreased oocyst production, enhanced body weight gain, and improved disease resistance following E. acervulina challenge infection (14, 18, 19). Covaccination with antigen plus the IFN-γ gene enhanced antibody titers in chickens (19), and coinjection of the IFN-γ gene with an Eimeria DNA vaccine improved protective immunity against coccidiosis (15).
In the present study, nine chicken cytokine genes were evaluated for their adjuvant effects on in ovo vaccination with the purified 3-1E recombinant protein against E. acervulina challenge infection. Five of the cytokines (IL-2, -15, -17, and -18 and IFN-γ) significantly reduced fecal-oocyst shedding when given in combination with 3-1E compared with 3-1E alone. In contrast, 3-1E plus IL-6 or -8 increased oocyst shedding to the level seen in the nonvaccinated and infected controls. Similarly, IL-16 or -18 or IFN-γ had a negative effect on body weight gain induced by 3-1E; these combinations led to weight gains equal to that seen in the nonvaccinated and infected group. As has been well documented in mammalian systems (2, 26), these results suggest that avian cytokines constitute complex biological networks with stimulatory and inhibitory effects produced by individual members to maintain overall homeostatic control. In addition, although no single parameter of coccidiosis is considered the “gold standard” for assessing disease morbidity, these results suggest that IL-2, -15, and -17 may be the most appropriate cytokines to use in combination with 3-1E in an in ovo delivery system for future vaccine trials. Current studies in our laboratory are directed toward this goal.
Acknowledgments
We are thankful for suggestions provided by Wongi Min and Rami Dalloul and the technical expertise of Diane Hawkins and Margie Nichols.
This project was supported by National Research Initiative grant 2004-01154 from the CSREES, United States Department of Agriculture.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Allen, P. C., and R. H. Fetterer. 2002. Recent advances in biology and immunobiology of Eimeria species and in diagnosis and control of infection with these coccidian parasites of poultry. Clin. Microbiol. Rev. 15:58-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arai, K. I., F. Lee, A. Miyajima, S. Miyatake, N. Arai, and T. Yokota. 1990. Cytokines: coordinators of immune and inflammatory responses. Annu. Rev. Biochem. 59:783-836. [DOI] [PubMed] [Google Scholar]
- 3.Bar-shira, E., D. Sklan, and A. Friedman. 2003. Establishment of immune competence in the avian GALT during the immediate post-hatch period. Dev. Comp. Immunol. 27:147-157. [DOI] [PubMed] [Google Scholar]
- 4.Brake, D. A. 2002. Vaccinology for control of apicomplexan parasites: a simplified language of immune programming and its use in vaccine design. Int. J. Parasitol. 32:509-515. [DOI] [PubMed] [Google Scholar]
- 5.Chapman, H. D., T. E. Cherry, H. D. Danforth, G. Richards, M. W. Shirley, and R. B. Williams. 2002. Sustainable coccidiosis control in poultry production: the role of live vaccines. Int. J. Parasitol. 32:617-629. [DOI] [PubMed] [Google Scholar]
- 6.Coletti, M., E. Del Rossi, M. P. Franciosini, F. Passamonti, G. Tacconi, and C. Marini. 2001. Efficacy and safety of an infectious bursal disease virus intermediate vaccine in ovo. Avian Dis. 45:1036-1043. [PubMed] [Google Scholar]
- 7.Hornok, S., Z. Szell, T. Sreter, A. Kovacs, and I. Varga. 2000. Influence of in ovo administered Cryptosporidium baileyi oocyst extract on the course of homologous infection. Vet. Parasitol. 89:313-319. [DOI] [PubMed] [Google Scholar]
- 8.Jakowlew, S. B., P. J. Dillard, P. Kondaiah, M. B. Sporn, and A. B. Roberts. 1988. Complementary deoxyribonucleic acid cloning of a novel transforming growth factor-beta messenger ribonucleic acid from chick embryo chondrocytes. Mol. Endocrinol. 2:747-755. [DOI] [PubMed] [Google Scholar]
- 9.Jenkins, M. C., M. D. Castle, and H. D. Danforth. 1991. Protective immunization against the intestinal parasite Eimeria acervulina with recombinant coccidial antigen. Poult. Sci. 70:539-547. [DOI] [PubMed] [Google Scholar]
- 10.Jeurissen, S. H., E. M. Janse, A. N. Vermeulen, and L. Vervelde. 1996. Eimeria tenella infections in chickens: aspects of host-parasite interaction. Vet. Immunol. Immunopathol. 54:231-238. [DOI] [PubMed] [Google Scholar]
- 11.Johnston, P. A., H. Liu, T. O'Connell, P. Phelps, M. Bland, J. Tyczkowski, A. Kemper, T. Harding, A. Avakian, E. Haddad, C. Whitfill, R. Gildersleeve, and A. Ricks. 1997. Applications in in ovo technology. Poult. Sci. 76:165-178. [DOI] [PubMed] [Google Scholar]
- 12.Kim, K. S., H. S. Lillehoj, and M. C. Jenkins. 1989. Evaluation of serum and secretory antibody responses to an immunodominant recombinant merozoite surface antigen, p150, using a sensitive enzyme-linked immunosorbent assay. Avian Dis. 33:431-437. [PubMed] [Google Scholar]
- 13.Kopko, S. H., D. S. Martin, and J. R. Barta. 2000. Responses of chickens to a recombinant refractile body antigen of Eimeria tenella administered using various immunizing strategies. Poult. Sci. 79:336-342. [DOI] [PubMed] [Google Scholar]
- 14.Lillehoj, H. S., and K. D. Choi. 1998. Recombinant chicken interferon-γ-mediated inhibition of Eimeria tenella development in vitro and reduction of oocyst production and body weight loss following Eimeria acervulina challenge infection. Avian Dis. 42:307-314. [PubMed] [Google Scholar]
- 15.Lillehoj, H. S., K. D. Choi, M. C. Jenkins, V. N. Vakharia, K. D. Song, J. Y. Han, and E. P. Lillehoj. 2000. A recombinant Eimeria protein inducing interferon-γ production: comparison of different gene expression systems and immunization strategies for vaccination against coccidiosis. Avian Dis. 44:379-389. [PubMed] [Google Scholar]
- 16.Lillehoj, H. S., and E. P. Lillehoj. 2000. Avian coccidiosis: a review of acquired intestinal immunity and vaccination strategies. Avian Dis. 44:408-425. [PubMed] [Google Scholar]
- 17.Lillehoj, H. S. 2003. Host immunity and vaccine development to coccidian and salmonella infections in chickens. J. Poult. Sci. 40:151-193. [Google Scholar]
- 18.Lowenthal, J. W., J. J. York, T. E. O'Neil, S. Rhodes, S. J. Prowse, D. G. Strom, and M. R. Digby. 1997. In vivo effects of chicken interferon-gamma during infection with Eimeria. J. Interferon Cytokine Res. 17:551-558. [DOI] [PubMed] [Google Scholar]
- 19.Lowenthal, J. W., J. J. York, T. E. O'Neil, R. A. Steven, D. G. Strom, and M. R. Digby. 1998. Potential use of cytokine therapy in poultry. Vet. Immunol. Immunopathol. 63:191-198. [DOI] [PubMed] [Google Scholar]
- 20.Lowenthal, J. W., T. E. O'Neil, D. G. Strom, and M. E. Andrew. 1999. Cytokine therapy: a natural alternative for disease control. Vet. Immunol. Immmuopathol. 72:183-188. [DOI] [PubMed] [Google Scholar]
- 21.Marsh, T. E., D. K. Fluke, and P. Villegas. 1997. Efficacy of INOVOJECT egg injection system for delivering Marek's disease vaccine under hatchery conditions. Avian Dis. 41:452-454. [PubMed] [Google Scholar]
- 22.Min, W., H. S. Lillehoj, J. Burnside, K. C. Weining, P. Staeheli, and J. J. Zhu. 2001. Adjuvant effects of IL-1β, IL-2, IL-8, IL-15, IFN-α, IFN-γ, TGF-β4 and lymphotactin on DNA vaccination against Eimeria acervulina. Vaccine 20:267-274. [DOI] [PubMed] [Google Scholar]
- 23.Min, W., and H. S. Lillehoj. 2002. Isolation and characterization of chicken interleukin-17 cDNA. J. Interferon Cytokine Res. 22:1123-1128. [DOI] [PubMed] [Google Scholar]
- 24.Min, W., and H. S. Lillehoj. 2004. Identification and characterization of chicken interleukin-16 cDNA. Dev. Comp. Immunol. 28:153-162. [DOI] [PubMed] [Google Scholar]
- 25.Nussler, A. K., and A. W. Thompson. 1992. Immunomodulatory agents in the laboratory and clinic. Parasitology 105:S5-S23. [DOI] [PubMed] [Google Scholar]
- 26.Pasquini, S., Z. Xiang, Y. Wang, Z. He, H. Deng, M. Blaszczyk-Thurin, and H. C. Ertl. 1997. Cytokine and costimulatory molecules as genetic adjuvants. Immunol. Cell. Biol. 75:397-401. [DOI] [PubMed] [Google Scholar]
- 27.Ramsay, J. A., J. A. Husband, and I. A. Ramshaw. 1994. The role of interleukin-6 in mucosal IgA antibody responses in vivo. Science 264:561-563. [DOI] [PubMed] [Google Scholar]
- 28.Reade, P. C., C. R. Jenkin, and K. J. Turner. 1965. The synthesis by fetal chicks and rats of serum proteins having some properties of the immunoglobulins. Aust. J. Exp. Biol. Med. Sci. 43:699-712. [DOI] [PubMed] [Google Scholar]
- 29.Reddy, D. N., P. G. Reddy, W. Xue, H. C. Minocha, M. J. Daley, and F. Blecha. 1993. Immunopotentiation of bovine respiratory disease virus vaccines by interleukin-1 beta and interleukin-2. Vet. Immunol. Immunopathol. 37:25-38. [DOI] [PubMed] [Google Scholar]
- 30.Reddy, S. K., J. M. Sharma, J. Ahmad, D. N. Reddy, J. K. McMillen, S. M. Cook, M. A. Wild, and R. D. Schwartz. 1996. Protective efficacy of a recombinant herpesvirus of turkeys as an in ovo vaccine against Newcastle and Marek's disease in specific-pathogen-free chickens. Vaccine 14:469-477. [DOI] [PubMed] [Google Scholar]
- 31.Ricks, C. A., A. Avakian, T. Bryan, R. Gildersleeve, E. Haddad, R. Ilich, S. King, L. Murray, P. Phelps, R. Poston, C. Whitfill, and C. Williams. 1999. In ovo vaccination technology. Adv. Vet. Med. 41:495-515. [PubMed] [Google Scholar]
- 32.Rossi, D., J. Sanchez-Garcia, W. T. McCormack, J. F. Bazan, and A. Zlotnik. 1999. Identification of a chicken “C” chemokine related to lymphotactin. J. Leukoc. Biol. 65:87-93. [DOI] [PubMed] [Google Scholar]
- 33.Schneider, K., F. Puehler, D. Baeuerle, S. Elvers, P. Staeheli, B. Kaspers, and K. C. Weining. 2000. cDNA cloning of biologically active chicken interleukin-18. J. Interferon Cytokine Res. 20:879-883. [DOI] [PubMed] [Google Scholar]
- 34.Sekellick, M. J., A. F. Ferrandino, D. A. Hopkins, and P. I. Marcus. 1994. Chicken interferon gene: cloning, expression, and analysis. J. Interferon Res. 14:71-79. [DOI] [PubMed] [Google Scholar]
- 35.Sharma, J. M., and B. R. Burmester. 1982. Resistance to Marek's disease at hatching in chickens vaccinated as embryos with the turkey herpesvirus. Avian Dis. 26:134-149. [PubMed] [Google Scholar]
- 36.Sharma, J. M., and R. L. Witter. 1983. Embryo vaccination against Marek's disease with serotypes 1, 2 and 3 vaccines administered singly or in combination. Avian Dis. 27:457-463. [PubMed] [Google Scholar]
- 37.Sharma, J. M. 1986. Embryo vaccination of specific-pathogen-free chickens with infectious bursal disease virus: tissue distribution of the vaccine virus and protection of hatched chickens against disease. Avian Dis. 30:776-780. [PubMed] [Google Scholar]
- 38.Sharma, J. M. 1987. Embryo vaccination of chickens with turkey herpesvirus: characteristics of the target cell of early viral replication in embryonic lung. Avian Dis. 16:367-379. [DOI] [PubMed] [Google Scholar]
- 39.Song, K. D., H. S. Lillehoj, K. D. Choi, C. H. Yun, M. S. Parcells, J. T. Huynh, and J. Y. Han. 2000. A DNA vaccine encoding a conserved Eimeria protein induces protective immunity against live Eimeria acervulina challenge. Vaccine 19:243-252. [DOI] [PubMed] [Google Scholar]
- 40.Tomley, F. M. 1994. Characterization of rhoptry proteins of Eimeria tenella sporozoites: antigenic diversity of rhoptry epitopes within species of the genus Eimeria and among three asexual generations of a single species, E. tenella. Infect. Immun. 62:4656-4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomley, F. M., J. M. Bumstead, K. J. Billington, and P. P. J. Dunn. 1996. Molecular cloning and characterization of a novel acidic microneme protein (Etmic-2) from the apicomplexan protozoan parasite, E. tenella. Mol. Biochem. Parasitol. 79:195-206. [DOI] [PubMed] [Google Scholar]
- 42.Vermeulen, A. N., J. J. Kok, P. van den Boogart, R. Dijkema, and J. A. J. Claessens. 1993. Eimeria refractile body proteins contain 2 potentially functional characteristics: transhydrogenase and carbohydrate transport. FEMS Microbiol. Lett. 100:223-229. [DOI] [PubMed] [Google Scholar]
- 43.Vermeulen, A. N., D. C. Schaap, and T. P. Schetters. 2001. Control of coccidiosis in chickens by vaccination. Vet. Parasitol. 100:13-20. [DOI] [PubMed] [Google Scholar]
- 44.Wakennell, P. S., J. M. Sharma, and R. F. Slocombe. 1995. Embryo vaccination of chickens with infectious bronchitis virus: histologic and ultrastructural lesion response and immunologic response. Avian Dis. 39:752-765. [PubMed] [Google Scholar]
- 45.Weber, F. H., and N. A. Evans. 2003. Immunization of broiler chicks by in ovo injection of Eimeria tenella sporozoites, sporocysts, or oocysts. Poult. Sci. 82:1701-1707. [DOI] [PubMed] [Google Scholar]
- 46.Weber, F. H., K. C. Genteman, M. A. LeMay, D. O. Lewis, and N. A. Evans. 2004. Immunization of broiler chicks by in ovo injection of infective stages of Eimeria. Poult. Sci. 83:392-399. [DOI] [PubMed] [Google Scholar]
- 47.Williams, R. B. 1999. A compartmentalized model for estimation of the cost of coccidiosis to the world's chicken production industry. Int. J. Parasitol. 29:1209-1229. [DOI] [PubMed] [Google Scholar]