Abstract
Antibody-dependent complement killing of Bordetella pertussis after immunization with a three-component acellular pertussis vaccine was characterized. Postimmunization activity was unchanged for about half of the adult vaccine recipients. The responses of the other individuals were complex, with evidence of both beneficial and antagonistic responses occurring, sometimes in the same individual.
We have examined complement killing of the respiratory pathogen Bordetella pertussis in adults immunized with a three-component acellular pertussis vaccine. B. pertussis has several defenses against the human complement system. The BrkA protein protects B. pertussis against the bactericidal activity of complement and antibody (3, 7). However, some individuals are able to mount an immune response that overcomes the BrkA defense, allowing activation of the antibody-dependent pathway of complement and culminating in bacterial death (19, 21). In a small study, individuals infected with B. pertussis were found to have a more potent bactericidal response than recipients of an acellular pertussis vaccine, and killing of wild-type B. pertussis was associated with an immunoglobulin G3 (IgG3) antibody response to lipopolysaccharide (21). In another study, bactericidal activity was assessed for paired pre- and postimmunization serum samples from recipients of several different acellular pertussis vaccines (19), and no improvement in bactericidal activity was noted after immunization.
Antibody-mediated complement killing of bacteria requires the proper antibody against an appropriate antigenic target. To generate the membrane attack complex, antigen on the surface of the bacteria must complex with complement-fixing antibody. IgG1 and IgG3 antibodies fix complement, while IgA antibodies do not. Furthermore, competition between IgA antibodies (which are not capable of activating complement) and IgG antibodies (which can activate complement) for binding to the target antigen has been shown to influence antibody-mediated complement-killing activity (11). Of the antigens in the three-component acellular vaccine, pertactin, an outer membrane protein, could most likely serve as a target for bactericidal activity, and mouse antibodies to pertactin have been shown to be bactericidal (9). Filamentous hemagglutinin (FHA) exists in both a membrane-bound and secreted form; therefore, only some molecules of FHA could serve as a target for complement-mediated killing. Similarly, pertussis toxin is secreted and could serve only as a transient target for complement-mediated killing. In this study, we wanted to determine whether an acellular pertussis vaccine would elicit antibody-mediated bactericidal activity in an adult population. We observed widely different responses in subjects receiving the same immunization.
Complement-mediated bactericidal killing.
Human serum depleted for immunoglobulin with intact complement activity was used as the source of complement. Antibody depletion was achieved by sequential incubations with agarose beads conjugated to protein G, protein L, and protein LA as previously described (2). Bacteria were grown under conditions that optimize expression of the complement resistance phenotype as previously described (3). Approximately 3 × 105 bacteria were added to wells in 96-well microtiter plates and incubated with heat-inactivated serum as a source of antibody at 37°C with shaking for 1 min to allow antibodies to bind. Complement was added to a concentration of 10%, and the bacteria were incubated for 2 h at 37°C. Serial dilutions of the bacterial suspensions were plated on Bordet Gengou agar (2). Relative survival was calculated by dividing the number of CFU from samples incubated with intact complement by the number of CFU from the negative-control samples, which were incubated with heat-inactivated serum lacking complement activity. Bactericidal activity is the inverse of relative survival. The mean bactericidal activity was determined from at least three independent trials for each serum sample at each dilution. Logarithmic values were compared using the Student t test. Each serum sample was tested in the absence of complement to ensure that it lacked activity. A pooled preparation of human serum plus complement served as a positive killing control, and antibody-depleted complement in the absence of added antibody served as a negative control.
Sera from vaccine recipients.
Serum samples were obtained from a subset of volunteers in a prospective randomized double-blind trial conducted at eight National Institutes of Health study sites in the United States over a 2-year period. Subjects from the Cincinnati, Ohio, site were recruited and randomized to receive either a three-component (FHA, pertussis toxoid, and pertactin) acellular pertussis vaccine lacking diphtheria and tetanus antigens, manufactured by GlaxoSmithKline (GSK), or a hepatitis A vaccine (Havrix; GSK). The adult vaccine, designed as a booster dose, contains one-third as much antigenic material as the GSK pediatric formulations, Infanrix and Pediarix (13).
We obtained 34 paired pre- and postimmunization serum samples as part of the blind study. Serum was collected prior to immunization and approximately 30 days later. The placebo group received hepatitis A vaccine (19 individuals), and the pertussis group (15 individuals) received the three-component (pertussis toxoid, FHA, and pertactin) acellular pertussis vaccine formulated for adults. Enzyme-linked immunoadsorbent assay (ELISA) was used to measure IgG and IgA antibodies as previously described (13). Purified protein (pertussis toxin, FHA, or pertactin) was obtained from GSK. The United States Food and Drug Administration reference serum (14), control serum, and subject serum specimens were added to each plate. The ELISA units were computed using UnitCalc software (Stockholm, Sweden) based on the reference line method.
Bactericidal activity.
As observed previously (19, 21), all of the adults in this study population had previous exposure to B. pertussis or cross-reacting antigens, as evidenced by bactericidal activity against the complement-sensitive BrkA mutant RFBP2152 (8) (data not shown). The ability to kill the wild-type, complement-resistant strain, BP338 (20), was used to assess the presence of bactericidal antibodies with the potential to mediate clearance of B. pertussis in vivo.
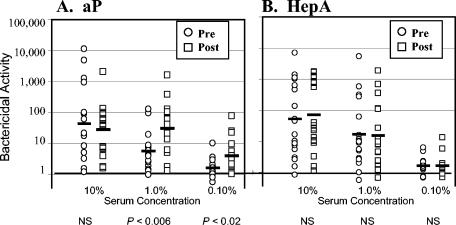
Bactericidal activity was determined for pre- and postimmunization sera. Three different concentrations of antisera were examined, with complement maintained at a constant concentration of 10%, which approximates the amount of complement on healthy human mucosal surfaces (4, 17). Bactericidal activity was plotted on a logarithmic scale, and the logarithmic mean values are shown in Fig. 1. The range of bactericidal activity prior to immunization was quite large and varied by more than 4 orders of magnitude for serum added at 10%.
FIG. 1.
Complement-mediated bactericidal activity pre- and postimmunization. Individuals immunized with the acellular pertussis (aP) vaccine (A) and individuals immunized with the hepatitis A (HepA) vaccine (B) are shown. Bactericidal activity for strain BP338 was determined for three different serum concentrations (10, 1.0, and 0.10%). Preimmunization values (circles), postimmunization values (squares), and geometric means (bars) are indicated. Pre- and postimmunization samples were compared using the Student t test, and the results are indicated below the each pair. NS, not statistically significant.
The preimmunization responses were compared to the postimmunization values using a paired t test (Fig. 1). Pre- and postimmunization responses were not different for individuals receiving the hepatitis A vaccine for any of the serum concentrations (Fig. 1B). For individuals receiving the pertussis vaccine (Fig. 1A), the preimmunization activity was not significantly different from the postimmunization activity when the highest serum concentration (10%) was tested. However, bactericidal activity increased significantly after immunization with the pertussis vaccine when the activity was compared using a more-dilute serum concentration of 1.0 or 0.1% (diluted serum) (P < 0.05). This supports the hypothesis that the pertussis vaccination was responsible for the altered immune responses.
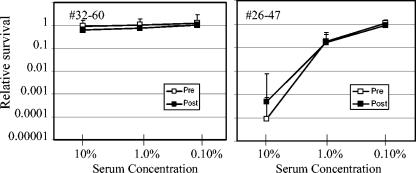
When examined individually, the pre- versus postimmunization bactericidal activity was not significantly different at any dilution tested for 8 of the 15 acellular vaccine recipients. The individuals in the group with unchanged activity after immunization included an individual with undetectable preimmunization activity against the wild-type strain (individual 32-60), and the individual with the highest preimmunization activity (individual 26-47) (Fig. 2). These results suggest that the level of preimmunization bactericidal activity does not necessarily influence the ability to generate a postimmunization response.
FIG. 2.
Subjects with unchanged bactericidal activity after immunization with acellular pertussis vaccine. Relative survival, or CFU after incubation in serum with 10% complement/CFU after incubation in serum without complement, of B. pertussis strain BP338 in the presence of various concentrations of pre- and postimmunization serum. Subject 32-60 is the individual with the lowest preimmunization bactericidal activity, and subject 26-47 is the individual with the highest preimmunization bactericidal activity.
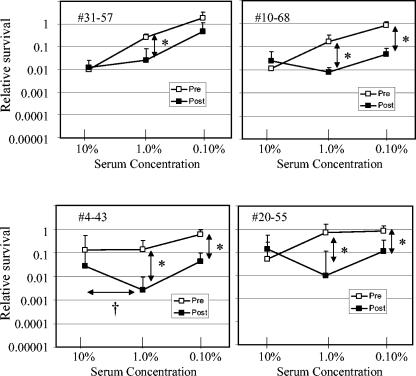
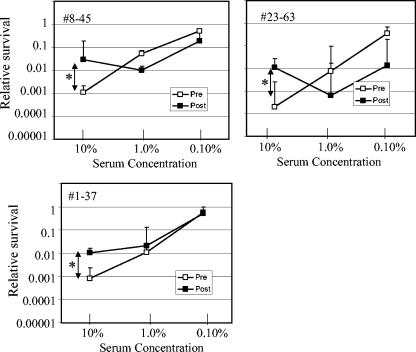
No change in bactericidal activity after immunization was observed when the serum was evaluated at a concentration of 10%. However, statistically significant differences between pre- and postimmunization bactericidal activity were observed (P < 0.05) using the paired t test for at least one serum dilution for 7 of the 15 acellular vaccine recipients. Furthermore, evidence of both improved bactericidal activity and reduced bactericidal activity after immunization was found in these seven serum samples. Four individuals displayed improved bactericidal activity after immunization when serum was added at lower concentrations (1.0 or 0.10%) but not at 10% (Fig. 3). However, in addition to enhanced bactericidal activity, individuals 4-43 and 20-55 displayed evidence of blocking activity, since fewer bacteria were killed when serum was added at 10% than when serum was added at 1%. Blocking activity could occur when antibodies that do not fix complement compete with complement-fixing antibodies for access to antigen. More definitive evidence of blocking was demonstrated in three other individuals (Fig. 4). For these individuals, the postimmunization serum samples had significantly less bactericidal activity than the preimmunization serum samples at a serum concentration of 10%. These results suggest some of the serum samples possessed a complement-blocking activity that was diminished upon dilution.
FIG. 3.
Subjects with enhanced bactericidal activity after immunization with acellular pertussis vaccine. Relative survival was determined as described in the legend to Fig. 2. Values were compared using the Student t test. Asterisks denote statistically significant differences (P < 0.05) for the pre- and postimmunization values indicated by the vertical arrows. A dagger denotes a statistically significant difference (P < 0.05) for the dilutions of postimmunization values indicated by the horizontal arrow.
FIG. 4.
Subjects with reduced bactericidal activity after immunization with acellular pertussis vaccine. Relative survival was determined as described in the legend to Fig. 2. Pre- and postimmunization values were compared using the Student t test. Asterisks denote statistically significant differences (P < 0.05) for the values indicated by the arrows.
IgG and IgA titers to vaccine antigens.
An ELISA was used to measure antibodies to pertussis toxin, FHA, and pertactin as previously described (16). All of the subjects receiving the acellular pertussis vaccine had increased IgG titers to each of the three vaccine antigens after immunization (Table 1), and many individuals had increased IgA titers. While all of the subjects displayed elevated antibody responses after vaccination, less than half of the individuals displayed altered bactericidal activity, suggesting that antibody titers determined by ELISA do not directly reflect bactericidal activity.
TABLE 1.
Pre- and postimmunization antibody titers sorted by bactericidal responsesa
| Group,b subject, or parameter | IgG titer to:
|
IgA titer to:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pertussis toxin
|
FHA
|
Pertactin
|
Pertussis toxin
|
FHA
|
Pertactin
|
|||||||
| Prec | Postd | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Group A | ||||||||||||
| 32-60 | 3 | 17 | 4 | 25 | 4 | 15 | 3 | 3 | 4 | 5 | 12 | 19 |
| 26-47 | 3 | 33 | 22 | 124 | 17 | 222 | 3 | 3 | 13 | 133 | 8 | 199 |
| 6-40 | 3 | 39 | 4 | 264 | 14 | 114 | 3 | 3 | 4 | 5 | 4 | 7 |
| 18-54 | 3 | 98 | 4 | 1,744 | 10 | 291 | 3 | 3 | 4 | 26 | 4 | 14 |
| 16-52 | 3 | 30 | 4 | 352 | 4 | 92 | 3 | 9 | 4 | 89 | 5 | 38 |
| 14-50 | 3 | 112 | 4 | 336 | 8 | 319 | 3 | 3 | 4 | 22 | 4 | 38 |
| 27-48 | 3 | 37 | 15 | 154 | 6 | 917 | 3 | 3 | 13 | 35 | 4 | 68 |
| 19-72 | 3 | 72 | 7 | 287 | 26 | 423 | 3 | 3 | 4 | 34 | 15 | 351 |
| GMTe | 3.0 | 45.7 | 6.3 | 228.6 | 9.1 | 183.0 | 3.0 | 3.4 | 5.4 | 25.9 | 6.1 | 43.5 |
| 95% CIf | 3.0g | 26.7-78.5 | 84.3-620 | 64.7-517 | 3.5-11.2 | 5.2-16.1 | 3.0 | 3.4-8.5 | 3.8-9.6 | 2.5-4.8 | 9.6-69.6 | 14.4-131 |
| Group B | ||||||||||||
| 31-57 | 3 | 48 | 4 | 936 | 30 | 349 | 3 | 5 | 32 | 1,308 | 9 | 212 |
| 10-68 | 8 | 124 | 25 | 754 | 13 | 1,746 | 3 | 7 | 45 | 2,051 | 9 | 257 |
| 4-43 | 3 | 166 | 9 | 580 | 11 | 1,525 | 3 | 3 | 4 | 55 | 4 | 56 |
| 20-55 | 3 | 132 | 4 | 342 | 12 | 1,553 | 3 | 6 | 4 | 13 | 5 | 163 |
| 23-63 | 3 | 58 | 32 | 376 | 42 | 2,520 | 3 | 19 | 14 | 252 | 179 | 1,843 |
| 8-45 | 3 | 69 | 9 | 660 | 17 | 2,224 | 3 | 5 | 5 | 567 | 8 | 1,828 |
| 1-37 | 3 | 20 | 68 | 158 | 77 | 398 | 3 | 3 | 37 | 68 | 18 | 71 |
| GMT | 3.5 | 72 | 13.2 | 475 | 22 | 1,182 | 3.0 | 5.6 | 12.9 | 211 | 12.3 | 275 |
| 95% CI | 2.4-4.9 | 37-142 | 4.9-36 | 272-832 | 11-44 | 559-2,500 | 3.0 | 3.2-10.0 | 4.7-35 | 38-1,160 | 3.8-40 | 75-101 |
Vaccine titers were significantly different for group A and group B, namely, preimmunization IgG titer to pertactin (P < 0.03), postimmunization IgG titer to pertactin (P < 0.005), IgA titer to FHA (P < 0.02), and IgA titer to pertactin (P < 0.03).
Group A is the group of subjects with unaltered bactericidal activity after immunization with pertussis vaccine. Group B is the group of subjects with altered bactericidal activity after immunization with pertussis vaccine.
Pre, preimmunization.
Post, postimmunization.
GMT, geometric mean titer.
95% CI, 95% confidence interval.
A value of 3.0 is shown when no variability was observed.
To understand the relationship between antibody responses and changes in bactericidal responses, the antibody titers for the subjects with unaltered bactericidal activity after immunization were compared to those of the subjects with altered bactericidal activity after immunization. The geometric mean preimmunization titers for the group with altered bactericidal activity (Table 1) were greater than those of the group with unaltered bactericidal activity, but only the preimmunization IgG titers to pertactin were statistically significantly different (P < 0.03 by Student's t test). In addition, postimmunization titers for the group with altered bactericidal activity were also greater than those of the group with unaltered bactericidal activity, and the postimmunization IgG titers to pertactin (P < 0.005), IgA titers to FHA (P < 0.02), and IgA titers to pertactin (P < 0.03) were statistically significant. The antibody titers for the individuals with blocking antibody responses (Fig. 4) were not significantly different from the individuals with improved bactericidal activity or from the group as a whole. The individuals with improved bactericidal activity (Fig. 3) had a higher geometric mean IgG titer to pertussis toxin than the other individuals did (P < 0.03).
The higher pre- and postimmunization titers in the group with altered bactericidal activity suggests that these individuals may have had recent exposure to B. pertussis and as a result mounted a stronger secondary immune response. In a study characterizing antibody responses after administration of the influenza vaccine, very high serum IgA and IgG responses were observed in primed individuals, or those with preimmunization serum antibodies, compared to unprimed individuals (6). The extremely high postimmunization IgA titers to the surface antigens, FHA, and pertactin in the group with altered bactericidal activity are particularly striking. Competition for binding to the bacteria between complement-activating antibodies (such as IgG1) and antibodies which are not capable of activating complement (such as IgA) can influence susceptibility to complement (11). Competition for antibody binding would occur only when the number of antibody molecules exceeds the number of antigenic sites, which could explain why increased bactericidal activity was observed more often with diluted serum.
For most of the subjects, we cannot determine whether the failure to observe increased bactericidal activity after immunization at high serum concentrations was due to the inability to generate antibodies that fix complement or the development of a blocking response. However, in one case (Fig. 3, 4-43), immunization appeared to induce the production of both complement-fixing antibodies (observed at low serum concentrations) and complement-blocking antibodies (observed at high serum concentrations), suggesting the concentrated serum possessed a complement-blocking activity that masked the complement-fixing activity observed in the diluted serum. Three other individuals displayed increased bactericidal activity only with diluted serum (Fig. 3), and perhaps these individuals also possessed a complement-blocking response that prevented increased killing at the highest serum concentration.
It is likely that B. pertussis has evolved to obstruct the generation of the immune response in a way that compromises the ability to effectively clear the microorganisms. The three antigens in the acellular pertussis vaccine (pertussis toxin, FHA, and pertactin), while not in their native conformation, are not necessarily inert protein antigens. All three proteins bind to receptors on the surfaces of human cells that mediate or modulate the immune response. Native pertussis toxin B-subunit can induce T-cell mitogenic activity in the absence of A-subunit enzymatic activity (10), and it can cause a reversal in the CD4+/CD8+ ratio in T cells cultured from lymph nodes (12). FHA can suppress interleukin-12 expression by macrophages, resulting in suppression of Th1-helper T-cell-mediated immune responses (15). Purified FHA has also been shown to induce apoptosis in macrophages (1). These activities could influence the class of antibody produced. IgA can be protective; it can block bacterial attachment to mucosal surfaces and limit bacterial colonization. However, antibodies that promote killing of the bacteria would be more protective than antibodies that reduce but do not eliminate colonization.
At least one of the antigens in the acellular pertussis vaccine appears to be able to serve as a target for complement-mediated bactericidal activity. However, in this study and other studies (19, 21), improved bactericidal responses after immunization were rarely observed, possibly due to induction of antibodies that fail to fix complement. The absence of vaccine-induced bactericidal activity in vitro is consistent with the observation that the pertussis vaccine is effective at preventing severe disease, likely due to pertussis toxin neutralization and blocking attachment to reduce bacterial colonization, but it is less effective at producing a sterilizing immune response (5, 18). Despite high vaccination rates, the number of reported cases of pertussis in the United States has increased steadily since the 1980s (22). Developing a pertussis vaccine with a greater potential to elicit bactericidal activity could reduce bacterial carriage and reduce the incidence of disease.
Acknowledgments
This work was supported in part by grants from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (grant RO1 AI45715 to A.A.W., grants NO1-AI-45252 and NO1-AI-2549 to D.I.B., and grants NO1-AI-25463 and NO-1-AI-45249 to J.I.W.).
Editor: J. T. Barbieri
REFERENCES
- 1.Abramson, T., H. Kedem, and D. A. Relman. 2001. Proinflammatory and proapoptotic activities associated with Bordetella pertussis filamentous hemagglutinin. Infect. Immun. 69:2650-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes, M. G., and A. A. Weiss. 2003. Activation of the complement cascade by Bordetella pertussis. FEMS Microbiol. Lett. 220:271-275. [DOI] [PubMed] [Google Scholar]
- 3.Barnes, M. G., and A. A. Weiss. 2001. BrkA protein of Bordetella pertussis inhibits the classical pathway of complement after C1 deposition. Infect. Immun. 69:3067-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandtzaeg, P. 1995. The role of humoral mucosal immunity in the induction and maintenance of chronic airway infections. Am. J. Respir. Crit. Care Med. 151:2081-2086. [DOI] [PubMed] [Google Scholar]
- 5.Cherry, J. D., J. Gornbein, U. Heininger, and K. Stehr. 1998. A search for serologic correlates of immunity to Bordetella pertussis cough illnesses. Vaccine 16:1901-1906. [DOI] [PubMed] [Google Scholar]
- 6.El-Madhun, A. S., R. J. Cox, and L. R. Haaheim. 1999. The effect of age and natural priming on the IgG and IgA subclass responses after parenteral influenza vaccination. J. Infect. Dis. 180:1356-1360. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez, R. C., and A. A. Weiss. 1994. Cloning and sequencing of a Bordetella pertussis serum resistance locus. Infect. Immun. 62:4727-4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez, R. C., and A. A. Weiss. 1998. Serum resistance in bvg-regulated mutants of Bordetella pertussis. FEMS Microbiol. Lett. 163:57-63. [DOI] [PubMed] [Google Scholar]
- 9.Gotto, J. W., T. Eckhardt, P. A. Reilly, J. V. Scott, J. L. Cowell, T. N. Metcalf III, K. Mountzouros, J. J. Gibbons, Jr., and M. Siegel. 1993. Biochemical and immunological properties of two forms of pertactin, the 69,000-molecular-weight outer membrane protein of Bordetella pertussis. Infect. Immun. 61:2211-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray, L. S., K. S. Huber, M. C. Gray, E. L. Hewlett, and V. H. Engelhard. 1989. Pertussis toxin effects on T lymphocytes are mediated through CD3 and not by pertussis toxin catalyzed modification of a G protein. J. Immunol. 142:1631-1638. [PubMed] [Google Scholar]
- 11.Griffiss, J. M., and D. K. Goroff. 1983. IgA blocks IgM and IgG-initiated immune lysis by separate molecular mechanisms. J. Immunol. 130:2882-2885. [PubMed] [Google Scholar]
- 12.Latif, R., N. K. de Rosbo, T. Amarant, R. Rappuoli, G. Sappler, and A. Ben-Nun. 2001. Reversal of the CD4+/CD8+ T-cell ratio in lymph node cells upon in vitro mitogenic stimulation by highly purified, water-soluble S3-S4 dimer of pertussis toxin. Infect. Immun. 69:3073-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le, T., J. D. Cherry, S. J. Chang, M. D. Knoll, M. L. Lee, S. Barenkamp, D. Bernstein, R. Edelman, K. M. Edwards, D. Greenberg, W. Keitel, J. Treanor, and J. I. Ward. 2004. Immune responses and antibody decay after immunization of adolescents and adults with an acellular pertussis vaccine: the APERT study. J. Infect. Dis. 190:535-544. [DOI] [PubMed] [Google Scholar]
- 14.Lynn, F., G. F. Reed, and B. D. Meade. 1996. Collaborative study for the evaluation of enzyme-linked immunosorbent assays used to measure human antibodies to Bordetella pertussis antigens. Clin. Diagn. Lab. Immunol. 3:689-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGuirk, P., and K. H. Mills. 2000. Direct anti-inflammatory effect of a bacterial virulence factor: IL-10-dependent suppression of IL-12 production by filamentous hemagglutinin from Bordetella pertussis. Eur. J. Immunol. 30:415-422. [DOI] [PubMed] [Google Scholar]
- 16.Millen, S. H., D. I. Bernstein, B. Connelly, J. I. Ward, S. J. Chang, and A. A. Weiss. 2004. Antibody-mediated neutralization of pertussis toxin-induced mitogenicity of human peripheral blood mononuclear cells. Infect. Immun. 72:615-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Persson, C. G., I. Erjefalt, U. Alkner, C. Baumgarten, L. Greiff, B. Gustafsson, A. Luts, U. Pipkorn, F. Sundler, and C. Svensson. 1991. Plasma exudation as a first line respiratory mucosal defence. Clin. Exp. Allergy 21:17-24. [DOI] [PubMed] [Google Scholar]
- 18.Storsaeter, J., H. O. Hallander, L. Gustafsson, and P. Olin. 1998. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine 16:1907-1916. [DOI] [PubMed] [Google Scholar]
- 19.Weingart, C. L., W. A. Keitel, K. M. Edwards, and A. A. Weiss. 2000. Characterization of bactericidal immune responses following vaccination with acellular pertussis vaccines in adults. Infect. Immun. 68:7175-7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss, A. A., and S. Falkow. 1983. Transposon insertion and subsequent donor formation promoted by Tn501 in Bordetella pertussis. J. Bacteriol. 153:304-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss, A. A., P. S. Mobberley, R. C. Fernandez, and C. M. Mink. 1999. Characterization of human bactericidal antibodies to Bordetella pertussis. Infect. Immun. 67:1424-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zanardi, L., F. B. Pascual, K. Bisgard, T. Murphy, M. Wharton, and E. Maurice. 2002. Pertussis—United States, 1997-2000. Morb. Mortal Wkly. Rep. 51:73-76. [PubMed] [Google Scholar]