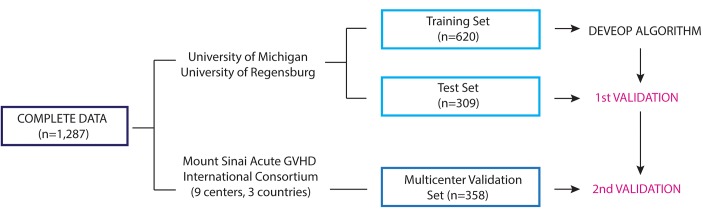
Figure 4. Study scheme of algorithm development and validation.
Clinical data and plasma samples from day 7 after hematopoietic cellular transplantation were available from 1,287 patients transplanted at 11 MAGIC centers. Patient samples from the 2 largest centers, the University of Michigan and the University of Regensburg, were randomly assigned to the training and test sets in a 2:1 proportion. The remaining 358 patients were assigned to the independent multicenter validation set. The training set alone (n = 620) was used to develop the algorithm. All possible combinations of 1 to 4 biomarkers were used to model 6-month nonrelapse mortality (NRM) by competing-risks regression. Rigorous comparison of models through a Monte Carlo cross validation of 75 different, randomly created training sets confirmed that the models using ST2 and REG3α were superior to all other biomarker combinations. We used this model to predict the probability of 6-month NRM in the patients from the training set, rank ordered them from lowest to highest, and chose a threshold to separate risk groups for the final algorithm (see Methods). We then applied the algorithm to the test set in a first validation and to the multicenter validation set in a second validation.