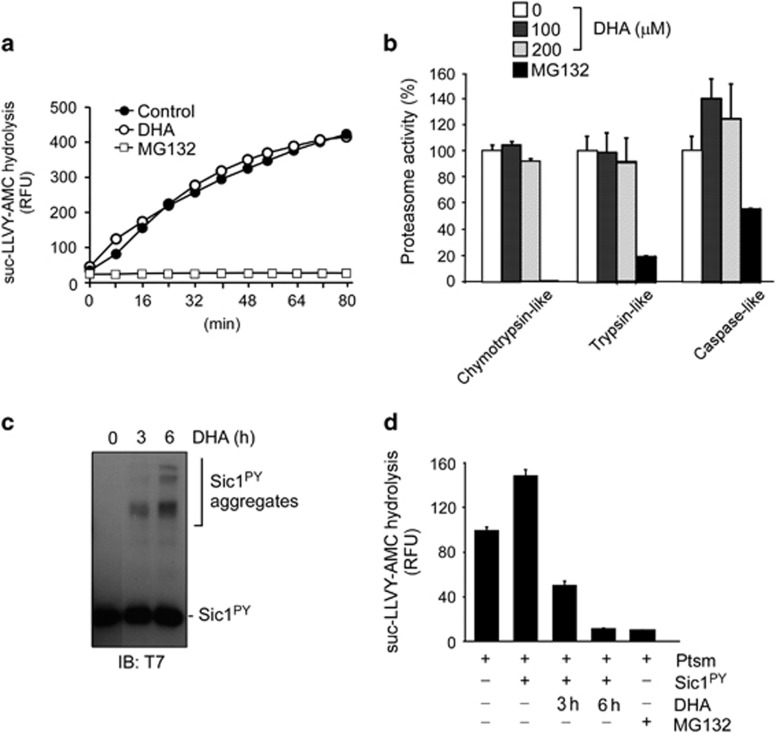
Figure 2.
Docosahexaenoic acid (DHA)-mediated protein aggregates, not DHA itself, inhibit proteasome activity. (a) Proteasome activity was kinetically monitored through hydrolysis of the fluorogenic peptides suc-LLVY-AMC (12.5 μM) in the presence of DHA (100 μM) or MG132 (10 μM). (b) The three different proteolytic sites in the purified human proteasome were measured by using the fluorogenic substrates suc-LLVY-AMC (for chymotrypsin-like activity), Boc-LRR-AMC (for trypsin-like) and Z-LLE-AMC (for caspase-like). DHA (0, 100 or 200 μM) or MG132 (10 μM) was added in the hydrolysis reactions, and relative proteasome activity was quantified after the reactions were quenched after 30 min. (c) In vitro Sic1PY (100 nM) aggregation mediated by DHA (200 μM for 3 or 6 h). T7-tagged Sic1PY proteins were analyzed by using SDS-PAGE/IB with T7 antibodies. (d) The effect of DHA-mediated aggregation of Sic1PY on proteasome activity was examined with suc-LLVY-AMC (12.5 μM) hydrolysis. Recombinant Sic1PY proteins (100 nM) were pre-incubated with 200 μM of DHA at 30 °C for 6 h and added to the hydrolysis reactions. MG132 (10 μM) was used, which indicated the baseline activity of purified proteasomes. RFU, relative fluorescence unit. Ptsm, 26S human proteasomes. Values represent means±s.d. (n=3).