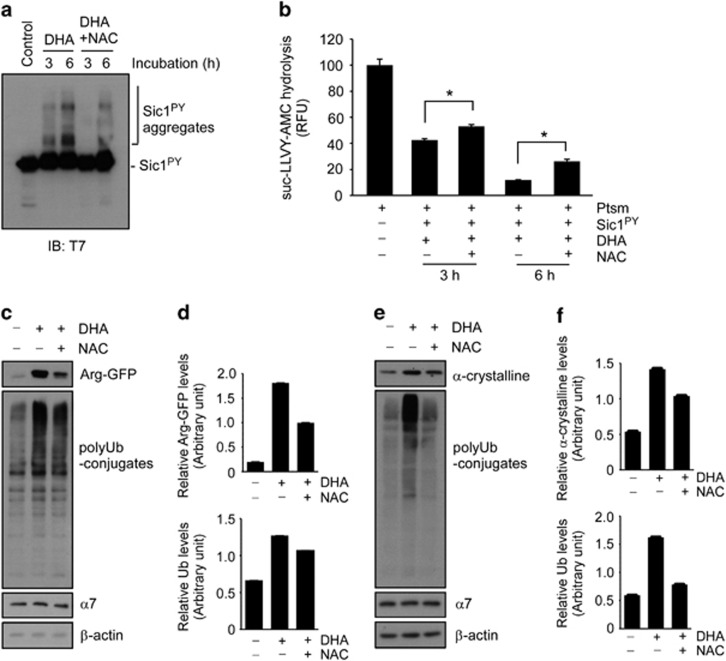
Figure 3.
An antioxidant reverses the inhibitory effects of docosahexaenoic acid (DHA) on proteasome activity by reducing protein aggregate formation. (a) DHA (200 μM) was co-incubated with recombinant Sic1PY (100 nM) in the presence or absence of N-acetyl cysteine (NAC, 200 μM) at 30 °C for the indicated time periods. Sic1PY aggregation was analyzed with SDS-PAGE/IB against T7 antibodies. (b) Proteasome activity was measured with suc-LLVY-AMC hydrolysis (for 30 min) in the presence of Sic1PY (100 nM), which was reacted with DHA (200 μM), NAC (200 μM), or a combination thereof for 3 or 6 h. Values represent means±s.d. *P<0.05 (n=3, ANOVA with Bonferroni's multiple comparison test). (c) HEK293 cells stably expressing Arg-GFP were treated with DHA (200 μM) and NAC (200 μM) for 4 h, and whole-cell extracts (WCEs) were prepared and analyzed with SDS-PAGE/IB using the antibodies indicated. (d) Quantification of c. (e) ARPE19 cells were transiently transfected with α-crystalline for 24 h and analyzed with SDS-PAGE/IB. (f) Quantification of α-crystalline or total polyUb conjugate levels in e.