Abstract
Recent in vitro and ex vivo studies disclosed an enhancement of the activity of antimicrobials on multidrug-resistant Pseudomonas aeruginosa by n-6 polyunsaturated fatty acids (PUFAS); therefore their effect was evaluated in experimental sepsis in 60 rabbits. Solutions of gamma-linolenic acid (GLA) and arachidonic acid (AA) were administered intravenously with ceftazidime and amikacin in rabbits with sepsis caused by one multidrug-resistant isolate. Therapy was started after bacterial challenge in five groups comprising 12 animals in each group: A, normal saline; B, antimicrobials; C, 99% ethanol and antimicrobials; D, GLA and antimicrobials; and E, AA and antimicrobials. Blood was sampled for the estimation of levels of endotoxins in serum (lipopolysaccharide), leukocytes, tumor necrosis factor alpha (TNF-α) and antimicrobials. Animals were sacrificed 210 min after bacterial challenge for tissue cultures. All animals had considerable endotoxemia and evolved leukopenia. The number of viable cells in blood, lung, and mesenteric lymph nodes was significantly reduced in groups D and E compared to that in other groups. Levels of antimicrobials in serum were inadequate to achieve bacterial killing due to the level of resistance. n-6 PUFAs did not influence TNF-α. It is concluded that intravenous coadministration of n-6 PUFAs and antimicrobials enhanced antimicrobial bacterial killing in experimental sepsis caused by multidrug-resistant P. aeruginosa.
Pseudomonas aeruginosa is a major nosocomial pathogen characterized by multidrug resistance. It has been previously shown by our study group that n-6 polyunsaturated fatty acids (PUFAs) like gamma-linolenic acid (C18:3; GLA) and arachidonic acid (C20:4; AA) may enhance the in vitro activity of ceftazidime and amikacin and render multidrug-resistant isolates susceptible to the combination of these antimicrobials (9). In proximity to in vitro findings, it was documented that serum sampled after the intravenous administration of one AA solution may ex vivo enhance the effect of ceftazidime and amikacin on multidrug-resistant P. aeruginosa (10).
Based on these previous findings, a solution of GLA and AA was administered intravenously with ceftazidime and amikacin for the therapy of an experimental model of sepsis caused by multidrug-resistant P. aeruginosa. The study attempted to simulate conditions of nosocomial sepsis treated by a combination of antimicrobials often inactive versus the causative pathogen (i.e., to a state where enhancement of the effect of antimicrobials is mandatory).
MATERIALS AND METHODS
Animals.
A total of 60 New Zealand White male rabbits of a mean (± standard deviation [SD]) weight of 3.39 ± 0.33 kg were studied. The study received a permit from the Veterinary Directorate of the Perfecture of Athens according to the Greek legislation in conformance with the 160/91 Directive Council of the European Union. Animals were housed in single metal cages and had access to tap water and standard balanced rabbit chow ad libitum. Room temperature ranged between 18 and 22°C, relative humidity ranged between 55 and 65%, and the light/dark cycle was 6 a.m./6 p.m.
Bacterial isolate.
One multidrug-resistant blood isolate of P. aeruginosa derived from a patient with nosocomial sepsis was studied. MICs of ticarcillin-clavulanate, piperacillin, ceftazidime, imipenem, meropenem, ciprofloxacin, and amikacin were determined by a microdilution technique at a 0.1-ml final volume. The MIC was considered the lowest concentrations of the tested antimicrobial limiting visible bacterial growth after 18 h of incubation at 35°C. Before each experiment, single colonies were incubated at 37°C in 10 ml of Mueller-Hinton broth (Oxoid Ltd., London, United Kingdom) for 8 h to yield a log-phase inoculum equal to 108 CFU/ml that was applied for bacterial challenge.
Preparation of the solutions of GLA and of AA.
Solutions of GLA and AA were prepared as reported previously (11). Briefly, n-6 PUFA ethyl esters (Sigma Co., St. Louis, Mo.) were dissolved in 99% ethanol (Merck, Darmstadt, Germany) to an initial dilution of 10 mg/ml. The dilution was refrigerated at −70°C under a nitrogen atmosphere, and appropriate amounts were removed from the refrigerator on the day of administration. n-6 PUFAs were administered at a dose of 25 mg/kg of animal weight (11). Respective amounts of the initial dilution of n-6 PUFAs were further diluted into sterile and endotoxin-free water (Difco Laboratories, London, United Kingdom) to a final volume of 50 ml and infused.
Study design.
Animals were initially sedated by intramuscular injection of 25-mg/kg ketamine and 5-mg/kg xylazine. They were then intubated and connected to a volume-controlled ventilator. Mechanical ventilation was adjusted to 16 breaths/min. Anesthesia was maintained by the intravenous administration of 20-mg/kg sodium thiopental. Through a middle line neck incision, both jugular veins and the left common carotid artery were dissected and catheterized by a 20-gauge catheter; catheters were stabilized with a 3.0 silk suture. A total of 108 CFU of the test isolate per kg was injected by the catheter inserted into the left jugular vein; the catheter remained in place for the entire experimental procedure. One animal was studied on each day of the experiment. Animals were then divided into the five study groups described below.
Group A.
Group A (n = 12) contained controls administered normal saline intravenously by the right jugular vein.
Group B.
In group B (n = 12), 30 minutes after bacterial challenge, 50-mg/kg ceftazidime (GlaxoWellcome, Macclesfield, United Kingdom) and 15-mg/kg amikacin (Bristol, Syracuse, N.Y.) were administered by the catheter of the right jugular vein. Ceftazidime, provided in one 2-g vial, was reconstituted with 40 ml of pyrogen-free water and infused within 5 min. Ready-to-use amikacin was given as a bolus. The applied doses of antimicrobials were selected in analogy to former studies (14).
Group C.
In group C (n = 12), 30 min after bacterial challenge, the respective amount of 99% ethanol diluted in water comprising the solutions of GLA and AA was infused by a pump within 15 min followed by both antimicrobials. The estimated amount of administered 99% ethanol was approximately 2 g/kg, which is lower than the dose reported to induce acute liver injury (15, 16).
Group D.
In group D (n = 12), 30 min after bacterial challenge, a 25-mg/kg solution of GLA was infused within 15 min by a pump connected to the catheter of the right jugular vein followed by both antimicrobials. The GLA regimen was selected in accordance with previous studies of our group on the 50% lethal dose of the applied solution (11).
Group E.
In group E (n = 12), 30 min after bacterial challenge, a 25-mg/kg solution of AA was infused within 15 min by a pump connected to the right jugular vein followed by both antimicrobials.
A volume of 5 ml of blood was sampled from the catheter of the left carotid before bacterial challenge and 30, 60, 120, 150, and 210 min after bacterial challenge. One milliliter of blood was added to 4 ml of Trypticase soy broth (Becton Dickinson) and applied for quantitative blood culture. The remainder was applied for the determination of the concentrations of endotoxins (LPS), tumor necrosis factor alpha (TNF-α), and antimicrobials. Blood was collected into pyrogen-free tubes (Vacutainer; Becton Dickinson, Cockeysville, Md.) and centrifuged; serum was kept refrigerated at −70°C until assayed. One more blood sample with a volume of 2 ml was collected from the catheter of the left carotid before bacterial challenge, and 210 min after bacterial challenge; it was collected in one EDTA-coated tube (Vacutainer) for estimation of the leukocyte count.
At 3.5 h (i.e., 3 h after the start of therapy), animals were sacrificed by the bolus intravenous administration of sodium thiopental. The time interval for animal sacrifice was selected on the grounds of providing evidence for a rapid, if any, effect on tissue bacterial counts. After a midline abdominal incision, segments of 0.3 to 0.5 g of liver, spleen, mesenteric lymph nodes, and of the lower lobe of the right lung were cut with separate blades; they were put into individual sterile containers and applied for quantitative culture.
Quantitative blood and tissue cultures.
The amount of blood added in broth was five times serially diluted 1:10 in 0.9% NaCl. A 0.1-ml aliquot of each dilution was plated onto MacConkey agar. The total amount of viable cells was estimated after reading their number on plates and multiplying by the appropriate dilution factor. The lower detection limit was 50 CFU/ml.
Tissue segments were weighted and homogenized; a 0.1-ml aliquot was then diluted 1:10 into sterile sodium chloride four consecutive times. Another aliquot of 0.1 ml of each dilution was plated onto MacConkey agar. Plates were incubated at 35°C for 48 h, and the number of viable colonies in each dilution was determined and multiplied by the appropriate dilution factor. Identification of colonies was performed with the API 20NE system (bioMérieux, Paris, France). The number of viable cells was expressed as CFU per gram. The lower detection limit was 10 CFU/g.
Assays for estimation of LPS, TNF-α, and leukocytes.
For the estimation of LPS, serum samples were diluted 1:10 in sterile and pyrogen-free water (BioWhitaker, Walkersville, Md.) and incubated for 5 min at 70°C. The concentration of LPS was then measured by the QCL-1000 Limulus amoebocyte lysate assay (BioWhitaker; measured in EU per milliliter, with a lower limit of detection of 1 endotoxin unit [EU]/ml) using a standard curve created by known concentrations of LPS of Escherichia coli serotype O111:B4. All determinations were performed in duplicate, and the mean of two observations was applied.
TNF-α was measured by a bioassay on the L929 fibrosarcoma cell line, as already described (4). Estimations involved all drawn samples: i.e., 360 assays. Briefly, confluent cells were thoroughly washed with Hanks solution (Biochrom AG, Berlin, Germany) and harvested with 0.25% trypsin-0.02% EDTA (Biochrom AG). Cells were centrifuged, resuspended in RMPI 1640 medium supplemented with 10% fetal bovine serum and 2 mM glutamine (Biochrom AG) and distributed into a 96-well cell culture plate at a density of 105 cells/well. The volume of fluid into each well was 0.05 ml. After incubation for 2 to 3 h at 37°C under 5% CO2, 0.06 ml of serum was added into each well followed by 0.05 ml of a 0.3-mg/ml dilution of cycloheximide (Sigma Co., St. Louis, Mo.). Known concentrations of human TNF-α (Sigma; range, 5.75 to 375.00 pg/ml) were added at the same time interval. Incubation continued overnight, and then the supernatant of each well was discarded and 0.1 ml of a 0.5-mg/ml methylene blue solution in 99% methanol was added. After 10 min, the dye was removed and the wells were thoroughly washed three times with 0.9% sodium chloride. The wells were left to dry, and remnants of the dye in each well became soluble by the addition of 0.1 ml of 50% glacial acetic acid (Merck, Darmstadt, Germany). The optical density in each well was read at 495 nm (Hitachi Spectrophotometer, Tokyo, Japan) against blank wells and control wells without added serum. Concentrations of TNF-α were estimated by the reduction of the optical density of control wells by unknown samples applying a standard curve generated by standard concentrations. All determinations were performed in quadruplicate. The interday variation of the assay was 13.75%.
The leukocyte count was estimated after passage of the EDTA-coated tube from a standard counter.
Pharmacokinetic study.
Estimation of levels of ceftazidime and amikacin in serum was performed by a microbiological assay, as already described (1), after application of two different reference strains: one resistant to amikacin and susceptible to β-lactams and one resistant to β-lactams and susceptible to amikacin. Mutants of E. coli ICB 4004 with resistance to amikacin (MIC of >1,028 μg/ml) were selected after serial passages of the parent strain onto MacConkey agar impregnated with increasing concentrations of amikacin; these colonies were applied for the determination of β-lactam levels. Bacillus subtilis ICB6633, which is resistant to ceftazidime and susceptible to amikacin, was applied for the determination of amikacin levels. All determinations were performed in triplicate, and their mean was applied. Concentrations were determined after drawing a standard curve with known concentrations of antimicrobials onto a semilogarithmic climax. All estimations were performed in triplicate; the lower detection limits were 0.35 μg/ml for ceftazidime and 0.7 μg/ml for amikacin. The standardized coefficients of the interday variation for the assay were 2% for ceftazidime and 0.1% for amikacin.
Statistical analysis.
The number of viable cells achieved 30 min after bacterial challenge was considered as the baseline of bacteremia; log10 changes from the baseline were estimated for each consecutive time interval. These changes were expressed by their mean (± standard error [SE]) and compared between groups for the same time interval.
Log10 estimates of viable cells of tissues were expressed as their mean (± SE) and compared between groups. Values of leukocytes were given as their mean (± SD). Levels of TNF-α and of antimicrobials in serum did not follow a normal distribution; they were given as their median and compared between groups for the same time interval.
Comparisons between groups were performed by analysis of variance with a correction according to Bonferroni. Any value of P < 0.05 was considered significant.
RESULTS
MICs of ticarcillin-clavulanate, piperacillin, ceftazidime, imipenem, meropenem, ciprofloxacin, and amikacin for the test isolate were >256/4, >256, 32, 16, 32, >256, and >512 μg/ml, respectively.
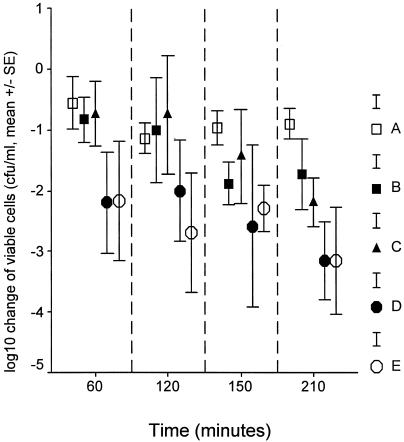
Viable counts of the test isolate in blood 30 min after bacterial challenge, when therapy was started, ranged between 5 × 105 and 1 × 106 CFU/ml. Changes in their number over time after application of treatment are given in Fig. 1. No statistically significant differences were found between groups A and C as well as between groups C and D or C and E. However, decreases in viable counts in groups D and E were significant compared to viable counts in group A at 210 min after bacterial challenge (for comparisons, P = 0.017 and 0.018, respectively).
FIG. 1.
Comparative reduction of viable blood cells from the baseline among the five treatment groups. Group A, controls; group B, antimicrobials; group C, 99% ethanol plus antimicrobials; group D, GLA plus antimicrobials; group E, AA plus antimicrobials. Therapy was administered 30 min after bacterial challenge, when the number of viable counts of the test isolate in blood ranged between 5 × 105 and 1 × 106 CFU/ml.
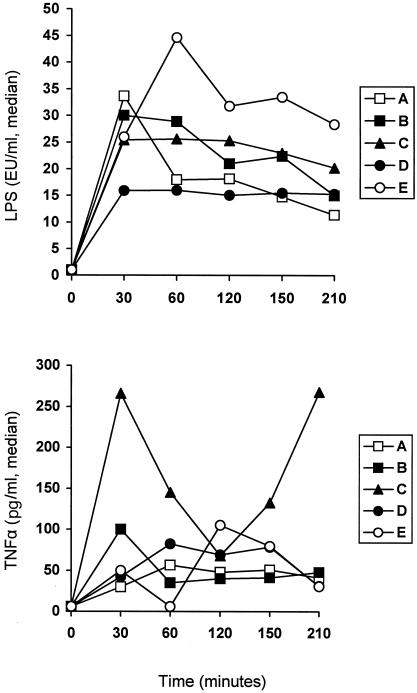
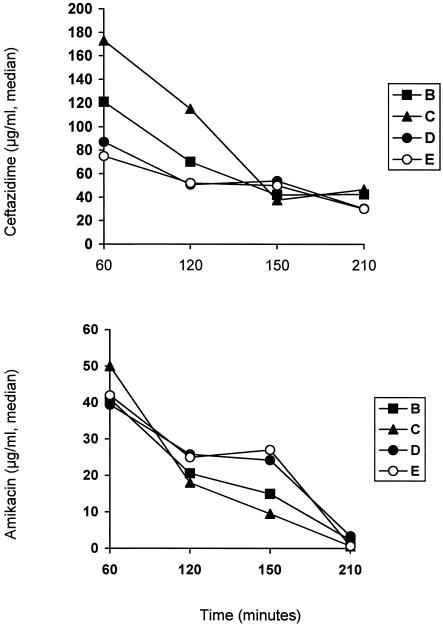
Concentrations of LPS and TNF-α in serum after bacterial challenge are given in Fig. 2; those of antimicrobials are shown in Fig. 3. No statistically significant differences were found between groups concerning levels of LPS and of TNF-α in serum as well as of ceftazidime and amikacin at any time interval. Levels of ceftazidime in serum were above the MIC for the test isolate for the entire 210-min period of follow-up. Those of amikacin were below the MIC for the test isolate.
FIG. 2.
Concentrations of endotoxins (LPS) and of TNF-α in serum over time after bacterial challenge. Group A, controls; group B, antimicrobials; group C, 99% ethanol plus antimicrobials; group D, GLA plus antimicrobials; group E, AA plus antimicrobials. Therapy was administered 30 min after bacterial challenge.
FIG. 3.
Concentrations of ceftazidime and amikacin in serum over time after bacterial challenge. Group B, antimicrobials; group C, 99% ethanol plus antimicrobials; group D, GLA plus antimicrobials; group E, AA plus antimicrobials. Therapy was administered 30 min after bacterial challenge.
The mean (± SD) leukocyte count of group A before bacterial challenge was 3,150.0 ± 1,484.9/μl, and it was changed to 2,066.7 ± 568.0/μl at 210 min after bacterial challenge (P 0.033). Respective values of group B were 3,950.0 ± 1,125.0 and 2,200.0 ± 1,119.5/μl (P = 0.003). Respective values of group C were 3,950.0 ± 925.0 and 2,100.0 ± 1,237.0/μl (P = 0.002). Respective values of group D were 5,600.0 ± 2,800 and 2,033.3 ± 647.0/μl (p: 0.001). Finally, the respective values of group E were 3,700.0 ± 565.6 and 1,960.0 ± 1006.5/μl (P = 0.001). No significant differences were found between groups concerning their leukocyte counts 210 min after bacterial challenge.
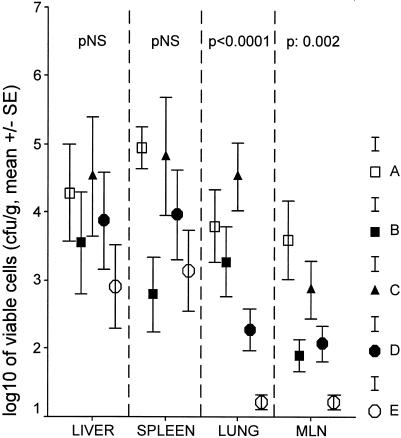
The bacterial loads in the liver, spleen, lungs, and mesenteric lymph nodes on animal sacrifice are given in Fig. 4. No differences were found between groups regarding liver and spleen isolates. Lung isolates of groups D or E were significantly lower than those of group C (for comparisons, P < 0.001 and P < 0.001, respectively). Levels of isolates of mesenteric lymph nodes of group D or E were lower than those of group C (for comparisons, P = 0.042 and P < 0.001 respectively). Similar differences were not found between groups C and A.
FIG. 4.
Bacterial loads in the liver, spleen, lower lobe of the right lung, and mesenteric lymph nodes (MLN) of each study group 210 min after bacterial challenge. Group A, controls; group B, antimicrobials; group C, 99% ethanol plus antimicrobials; group D, GLA plus antimicrobials; group E, AA plus antimicrobials.
DISCUSSION
There is striking evidence that dietary supplementation with PUFAs is a probable candidate for immunomodulatory therapy due to PUFAs' effect on lymphocyte and monocyte functions (2). These data are based on experimental studies with long-term animal feeding diets enriched with PUFAs, so it has become doubtful whether the described model would fit an acute inflammatory state like sepsis. Recent evidence suggested that n-6 PUFAs enhanced both in vitro and ex vivo the effect of antimicrobials on multidrug-resistant nosocomial isolates (9, 10). The present study attempted to evaluate the effect of n-6 PUFAs on the management of sepsis by nosocomial multidrug-resistant P. aeruginosa. Simulation of the studied animal model for clinical practice is based on the empirical application of a combination of antimicrobials in nosocomial sepsis, where antimicrobials are often inactive against the causative pathogen.
The applied experimental model successfully mimicked sepsis, as documented by high levels of endotoxemia (Fig. 1) accompanied by leukopenia. Coadministration of n-6 PUFAs and antimicrobials achieved a rapid decrease in viable cells in blood, reaching a peak 210 min after bacterial challenge (Fig. 1). At the same time interval, similar decreases were found for the bacterial load in the lungs and mesenteric lymph nodes (Fig. 4). The decrease in viable cells achieved after the addition of n-6 PUFAs was greater than 3 log10, which is considered the criterion for a bactericidal effect (9). A similar effect was not disclosed by the single application of antimicrobials in either blood or tissue cell counts of the test pathogen.
Special reference should be made to the pharmacokinetic-pharmacodynamic relationship for ceftazidime and amikacin in the groups of treatment. It is known that the main pharmacokinetic parameter determining in vivo bacterial killing for a β-lactam agent is time above the MIC (12). In the present study, applied doses of ceftazidime conferred constant serum drug levels above the MIC (Fig. 2). Amikacin is known to exert concentration-dependent bacterial killing based on the achievement of serum drug concentrations much higher than the MIC (12). That criterion was not fulfilled in the present study.
Based on the above observations, it might be stated that the presence of n-6 PUFAs enhanced the activity of antimicrobials on multidrug-resistant P. aeruginosa for the following reasons: (i) single n-6 PUFAs have been shown to induce transient decreases of in vitro bacterial growth and not any bactericidal effect (7); (ii) decreases in blood cells by ceftazidime and amikacin at 210 min reached statistical significance only after addition of n-6 PUFAs (Fig. 1), and (iii) the pharmacokinetic-pharmacodynamic relationship for ceftazidime and amikacin was the same for groups B, C, D, and E. Decreases in bacterial counts were detected only in groups D and E where n-6 PUFAs were applied.
The mechanism of action of n-6 PUFAs might involve either interference with the inflammatory cascade or a direct effect on the bacterial cell. Reports involving an animal model of sepsis underscore the anti-inflammatory potential of PUFAs expressed by a reduction of serum cytokine levels and of superoxide formation (2, 3, 5). Interference with the inflammatory cascade is attributed to alterations of the biosynthesis of prostaglandins and subsequently of TNF-α. In these models, sepsis was induced several weeks after dietary supplementation with diets enriched in PUFAs, so that a considerable period was provided for any metabolic interventions to occur. In the present study, GLA or AA was administered intravenously after bacterial challenge, so that the provided interval for involvement into metabolic pathways was not adequate. TNF-α biosynthesis did not seem to become influenced (Fig. 2). Addition of PUFAs did not prevent leukopenia from evolving, representing another piece of evidence against probable involvement of PUFAs with the inflammatory cascade in the present study.
In vitro evidence (8) suggests a direct effect of n-6 PUFAs on bacterial cells. n-6 PUFAs are prone to peroxidation ending in free radicals capable of attacking bacterial outer membrane and facilitating the action of antimicrobials. Their effect might also be accentuated by their beneficial effect on phagocytosis by the host (6, 13).
To the best of our knowledge, this is the first study attempting to administer intravenously a solution of a single n-6 PUFA, GLA or AA, for the therapy of sepsis by nosocomial multidrug-resistant P. aeruginosa. It was clearly demonstrated that infusion of n-6 PUFAs enhanced the activity of antimicrobials leading to reduction of viable cells in blood and tissues. These findings, in conjunction with previous reports that n-6 PUFAs might exert an inhibitory effect on growth of susceptible E. coli and P. aeruginosa (7, 8), constitute an indication for the application of single n-6 PUFas, instead of diets enriched in PUFAs, as enhancers of antimicrobial chemotherapy. n-6 PUFAs failed to induce any anti-inflammatory effect. However, the mainstay for the management of nosocomial sepsis is bacterial eradication. In that sense, coadministration of n-6 PUFAs with antimicrobials is a promising perspective for the management of nosocomial sepsis caused by multidrug-resistant pathogens.
REFERENCES
- 1.Adamis, G., M. G. Papaioannou, E. J. Giamarellos-Bourboulis, P. Gargalianos, J. Kosmidis, and H. Giamarellou. 2004. Pharmacokinetic interactions of ceftazidime, imipenem and aztreonam with amikacin in healthy volunteers. Int. J. Antimicrob. Agents 23:144-149. [DOI] [PubMed] [Google Scholar]
- 2.Calder, P. C. 2001. Polyunsaturated fatty acids, inflammation and immunity. Lipids 36:1007-1024. [DOI] [PubMed] [Google Scholar]
- 3.Chyi, A. C., and S. L. Yeh. 2000. Effects of dietary fish oil on survival rate, plasma amino acid pattern, and inflammatory-related mediators in diabetic rats with sepsis. Clin. Nutr. 19:313-318. [DOI] [PubMed] [Google Scholar]
- 4.Engelhard, D., S. Pomeranz, S. Gallily, N. Strauss, and E. Tuomanen. 1997. Serotype-related differences in inflammatory response to Streptococcus pneumoniae in experimental meningitis. J. Infect. Dis. 175:979-982. [DOI] [PubMed] [Google Scholar]
- 5.Fritsche, K. L., M. Byrge, and C. Feng. 1999. Dietary omega-3 polyunsaturated fatty acids from fish oil reduce interleukin-12 and interferon-gamma production in mice. Immunol. Lett. 65:167-173. [DOI] [PubMed] [Google Scholar]
- 6.Garnacho-Montero, J., C. Ortiz-Leyba, C. Garnacho-Montero, J. L. Garcia-Garmendia, C. Pérez-Paredes, M. R. Moyano-Del Estad, A. Barrero-Almodóvar, and F. J. Jiménez-Jiménez. 2002. Effects of three intravenous lipid emulsions on the survival and mononuclear phagocyte function of septic rats. Nutrition 18:751-754. [DOI] [PubMed] [Google Scholar]
- 7.Giamarellos-Bourboulis, E. J., P. Grecka, A. Dionyssiou-Asteriou, and H. Giamarellou. 1995. In vitro inhibitory activity of gamma-linolenic acid on Escherichia coli strains and its influence on their susceptibilities to various antimicrobial agents. J. Antimicrob. Chemother. 36:327-334. [DOI] [PubMed] [Google Scholar]
- 8.Giamarellos-Bourboulis, E. J., P. Grecka, A. Dionyssiou-Asteriou, and H. Giamarellou. 1998. In vitro activity of polyunsaturated fatty acids on Pseudomonas aeruginosa: relationship to lipid peroxidation. Prostaglandins Leukot. Essent. Fatty Acids 58:283-287. [DOI] [PubMed] [Google Scholar]
- 9.Giamarellos-Bourboulis, E. J., P. Grecka, A. Dionyssiou-Asteriou, and H. Giamarellou. 2000. Impact of n-6 polyunsaturated fatty acids on growth of multidrug-resistant Pseudomonas aeruginosa: interactions with amikacin and ceftazidime. Antimicrob. Agents Chemother. 44:2187-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giamarellos-Bourboulis, E. J., D. Plachouras, S. Skiathitis, M. Raftogiannis, A. Dionyssiou-Asteriou, I. Dontas, P. E. Karayannacos, and H. Giamarellou. 2003. Ex vivo synergy of arachidonate-enriched serum with ceftazidime and amikacin on multidrug-resistant Pseudomonas aeruginosa. J. Antimicrob. Chemother. 51:423-426. [DOI] [PubMed] [Google Scholar]
- 11.Giamarellos-Bourboulis, E. J., S. Skiathitis, A. Dionyssiou-Asteriou, I. Dontas, S. Hatziantoniou, K. Demetzos, G. T. Papaioannou, G. Karatzas, and H. Giamarellou. 2002. Rapid alterations of serum oxidant and antioxidant status with the intravenous administration of n-6 polyunsaturated fatty acids. Prostaglandins Leukot. Essent. Fatty Acids 67:57-62. [DOI] [PubMed] [Google Scholar]
- 12.Periti, P., and T. Mazzei. 1998. Antibiotic-induced release of bacterial cell wall components in the pathogenesis of sepsis and septic shock: a review. J. Chemother. 10:427-448. [DOI] [PubMed] [Google Scholar]
- 13.Puertollano, M. A., M. A. de Pablo, and G. Álvarez de Cienfuegos. 2001. Immunomodulatory effects of dietary lipids alter host natural resistance of mice to Listeria monocytogenes infection. FEMS Immunol. Med. Microbiol. 32:47-52. [DOI] [PubMed] [Google Scholar]
- 14.Robaux, M. A., L. Dube, J. Caillon, D. Bugnon, M. F. Kergueris, D. Navas, F. Le Conte, D. Barron, and G. Potel. 2001. In vivo efficacy of continuous infusion versus intermittent dosing of ceftazidime alone or in combination with amikacin relative to human kinetic profiles in a Pseudomonas aeruginosa rabbit endocarditis model. J. Antimicrob. Chemother. 47:617-622. [DOI] [PubMed] [Google Scholar]
- 15.Yang, R., X. Han, R. L. Delude, and M. P. Fink. 2003. Ethyl pyruvate ameliorates acute alcohol-induced liver injury and inflammation in mice. J. Lab. Clin. Med. 142:285-287. [DOI] [PubMed] [Google Scholar]
- 16.Zhou, Z., L. Wang, Z. Song, J. C. Lambert, C. J. McClain, and Y. J. Kang. 2003. A critical involvement of oxidative stress in acute alcohol-induced hepatic TNF-alpha production. Am. J. Pathol. 163:1137-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]