Abstract
β-d-N4-Hydroxycytidine (NHC) was found to have selective anti-hepatitis C virus (HCV) activity in the HCV replicon system (clone A). The intracellular metabolism of tritiated NHC was investigated in the HCV replicon system, Huh-7 cells, HepG2 cells, and primary human hepatocytes. Incubation of cells with 10 μM radiolabeled NHC demonstrated extensive and rapid phosphorylation in all liver cells. Besides the 5′-mono, -di-, and -triphosphate metabolites of NHC, other metabolites were characterized. These included cytidine and uridine mono-, di-, and triphosphates. UTP was the predominant early metabolite in Huh-7 cells and primary human hepatocytes, suggesting deamination of NHC as the primary catabolic pathway. The intracellular half-lives of radiolabeled NHC-triphosphate and of CTP and UTP derived from NHC incubation in Huh-7 cells were calculated to be 3.0 ± 1.3, 10.4 ± 3.3, and 13.2 ± 3.5 h (means ± standard deviations), respectively. Studies using monkey and human whole blood demonstrated more-rapid deamination and oxidation in monkey cells than in human cells, suggesting that NHC may not persist long enough in plasma to be delivered to liver cells.
Hepatitis C virus (HCV) causes liver disease and is spread primarily by contact with the blood of an infected person. Globally, an estimated 170 million persons are chronically infected with HCV (20). An estimated 3.9 million Americans (1.8%) have been infected with HCV, and cirrhosis will eventually develop in at least 15 to 20 percent of them (1, 12). HCV is one of the most important causes of chronic liver disease and seems to progress more rapidly to liver damage in human immunodeficiency virus-infected persons than in noninfected persons (13). Interferon, alone and in combination with ribavirin, is approved for the treatment of persons with chronic hepatitis C. Treatment with interferon for genotype 1-infected individuals is effective in about 15 to 20% of patients (6, 16), but when combined with ribavirin, its effectiveness increases to almost 42%; ribavirin alone, however, is ineffective (6). The combination of ribavirin and interferon is 80% effective against genotypes 2 and 3 (12, 16). Therefore, we need to develop new antiviral agents active against all genotypes of HCV, particularly genotype 1, which is commonly found in the United States and China (11).
β-d-N4-Hydroxycytidine (NHC), a base-modified ribonucleoside analogue, was identified as a potent and selective anti-HCV candidate (19). In a bovine viral diarrhea virus infection system and in HCV replicon RNA in Huh-7 cells, NHC had 90% effective concentrations (EC90) of 2 and 5 μM, respectively (19). NHC was nontoxic (50% inhibitory concentration, >100 μM) in the HCV replicon system (clone A cells, genotype 1b), Huh-7 cells, HepG2 cells, and human peripheral blood mononuclear cells (19). Levels of mitochondrial DNA and RNA or lactic acid in HepG2 cells treated with NHC did not change, suggesting no delayed toxicity (19). Although the antiviral mechanism of action of NHC is not completely understood, its nucleotide may act as a weak alternative substrate for CTP in the HCV RNA polymerization reaction (19). Based on studies with the viral RNA polymerase, we speculate that the incorporation of NHC-5′-monophosphate (NHC-MP) into viral RNA has the capacity to change the thermodynamics of regulatory secondary structures (with or without introducing mutations) and that nucleoside analogues such as NHC may represent an important class of new antiviral agents for the treatment of RNA virus infections, especially HCV (19).
In order to advance its preclinical development, it was important to investigate the metabolism of [3H]NHC in various liver cells, including clone A cells, Huh-7 cells, HepG2 cells, and primary human hepatocytes. This could provide insight into its metabolism and the intracellular half-life (t1/2) of the active metabolite NHC-5′-triphosphate (NHC-TP). We also determined the stability of NHC in monkey and human whole blood as a prelude to studies with primates.
MATERIALS AND METHODS
Chemicals and supplies.
NHC used in this study was synthesized by C. K. Chu (University of Georgia, Athens). [5-3H]NHC (13.0 Ci/mmol) was custom synthesized from cytidine by catalytic tritium exchange by Moravek Biochemicals, Inc. (Brea, Calif.). NHC-TP was synthesized as described previously (19). Tetrabutylammonium phosphate (TBAP) was purchased from Alltech Associates Inc. (Deerfield, Ill.). The scintillation liquid EcoLite was obtained from Valeant Pharmaceuticals International (Costa Mesa, Calif.). All other chemicals were obtained from Sigma Chemical Co. (St. Louis, Mo.). Fresh monkey EDTA blood was kindly provided by H. McClure (Yerkes National Primate Research Center, Emory University, Atlanta, Ga.), and human EDTA blood was obtained by venipuncture from healthy volunteers. CTP, UTP, and the N′,N′-dimethylhexylamine (DMHA) were purchased from Sigma-Aldrich (Milwaukee, Wis.). Acetonitrile and acetic acid were purchased from Fisher (Fair Lawn, N.J.), and deionized water was obtained from a Modulab 2020 system.
Cell culture systems.
The human hepatocellular carcinoma cell lines HepG2 and Huh-7 were obtained from the American Type Culture Collection (Manassas, Va.) and maintained in a 75-cm2 flask in Dulbecco's modified Eagle's medium supplemented with 4.5 g of glucose/liter and sodium pyruvate (MediaTech Inc., Herndon, Va.), 10% (vol/vol) heat-inactivated fetal bovine serum, and 1 mM penicillin G-streptomycin sulfate. All cell lines were grown at 37°C in a 5% CO2-95% air atmosphere. The medium was replenished every 3 days, and cells were subcultured once a week.
Primary human hepatocytes and medium were obtained from In Vitro Technologies (Baltimore, Md.). The HCV replicon RNA clone A system in Huh-7 cells was provided by Apath, LLC (St. Louis, Mo.).
Digestion of cellular extracts with alkaline phosphatase.
HepG2 cells (1 × 106 to 1.8 × 106 per well) were resuspended in a final volume of 1.5 ml per time period and exposed to 10 μM [3H]NHC (500 dpm/pmol) for specific time periods. The cells were maintained at 37°C under a 5% CO2-95% air atmosphere. Intracellular metabolites were extracted as described below. Cell extracts from HepG2 cells that had been incubated with tritiated NHC were treated with 1.0 U of alkaline phosphatase per 106 cells (cat. no. P-6772; Sigma) at 37°C overnight. The samples were analyzed by reverse-phase high-performance liquid chromatography (HPLC).
LC-tandem MS (MS-MS) system conditions.
Nucleotides are not expected to be retained on conventional reverse-phase HPLC columns. To obtain a good separation, phosphate buffer (4, 5, 9) or ion-exchange chromatography (18) is used in LC-UV visible light detection of nucleotides. However, in electrospray ionization-mass spectrometry (MS), a low concentration of nonvolatile salts can markedly decrease the MS sensitivity and even prevent ionization (7). Therefore, the challenge was to select an ion-pairing agent that did not cause interference in MS detection of the nucleotides. Thus, the first criterion in selecting an appropriate ion-pairing agent was to ensure that the nucleotides were retained and separated on a reversed-phase HPLC column. According to the literature (2, 8, 17), the best results for ion pairing are obtained with DMHA. First, the pure compounds were infused into the eluent mixture (ratio of mobile phase A to mobile phase B, 40:60 [vol/vol]; 1 μg/ml) at 10 ml/min to determine the optimum ionization mode. As expected for the nucleotide studies, the negative mode was chosen.
We used a Hypersyl BDS C8 column (4.6 by 150 mm, 5 μm; Phenomenex, Torrance, Calif.) as the analytical column and guard pack precolumn. A Waters LC system (controller 600 and UV photodiode array detector 996) was used for this analysis. Mobile phase A was composed of acetonitrile-water (8/2) and had a pH of 7 (adjusted with 5 mM DMHA and acetic acid), and mobile phase B was composed of water and 25 mM DMHA and had a pH of 7 (adjusted with acetic acid). Forty microliters of each sample was injected by use of an autosampler (model 717; Waters). Elution was performed using a linear gradient from 0 min (with A at 5%) to 5 min (with A at 10%), 5 to 30 min (with A at 30%), and 30 to 40 min (with A at 40%) at a flow rate of 1 ml/min.
The MS system used was a Finnigan TSQ 7000 (ThermoFinnigan, San Jose, Calif.), operating in electrospray ionization negative-ion mode. The MS parameters were optimized on the pure CTP, UTP, and NHC-TP. Nitrogen was used as both the sheath and auxiliary gases at pressures of 80 and 20 U, respectively. The spray voltage and the capillary temperature were set at 4.5 kV and 300°C, respectively. Collision-induced dissociation (CID) of the parent ions was performed in the collision cell (Q2) with argon gas at 2 torr and with collision energy optimized for each compound, producing the highest abundance of product ions in Q3. Because a high concentration of DMHA was used, the MS source and the whole LC column were regularly cleaned with eluent at the end of each day with 50/50 water-methanol.
Nucleoside accumulation studies.
For these studies, Huh-7 cells, clone A cells, HepG2 cells, and primary human hepatocytes (1.5 × 106 to 2.5 × 106 per well in a six-well plate) were resuspended in a final volume of 1.5 ml of culture medium per time period and exposed to 10 μM [3H]NHC (500 dpm/pmol) for specific time periods (1, 2, 4, 8, and 24 h). The cells were maintained at 37°C under a 5% CO2-95% air atmosphere. Intracellular metabolites were extracted as described below.
Determination of intracellular NHC-TP t1/2s.
Huh-7 cells (2.5 × 106 per well of a six-well plate) were incubated with 10 μM [3H]NHC (500 dpm/pmol) for a period of 24 h at 37°C in a 5% CO2 atmosphere. The cells were then washed three times with drug-free medium to remove extracellular NHC and incubated with regular culture medium for specific time periods (0, 1, 2, 4, 8, and 24 h). Intracellular metabolites were extracted as described below.
Determination of intracellular metabolites.
At selected times for NHC-TP accumulation or for the determination of NHC-TP t1/2 study time points, extracellular medium was removed and the cell layer was washed with cold phosphate-buffered saline. After cell scraping with 60% methanol (1 ml), NHC and its respective metabolites were extracted by incubation overnight at −20°C, and then samples were centrifuged at 14,000 rpm (Eppendorf centrifuge model 5415C) for 5 min and supernatant was collected. This was followed by extraction for 1 h on ice the next day (200 μl with 60% methanol), and samples were centrifuged again at 14,000 rpm (Eppendorf centrifuge model 5415C) for 5 min. Extracts were combined, dried under a gentle filtered airflow, and then stored at −20°C until they were analyzed by HPLC. Residues were resuspended in 200 μl of water, and aliquots were injected into the HPLC column.
Stability study of NHC in monkey and human whole blood.
Ten micromolar [3H]NHC (1,000 dpm/pmol) was incubated in either monkey or human blood for different periods of time (0, 0.08, 0.16, 1, 2, 4, and 24 h). At a selected time point, an aliquot of 200 μl was taken and centrifuged at 14,000 rpm (Eppendorf centrifuge model 5415C) for 5 min. The supernatant was collected, and 500 μl of acetonitrile was added and mixed. The sample was recentrifuged at 14,000 rpm for 5 min, and the supernatant was dried using a DNA speed vacuum (model DNA 110; Savant Instrumental Inc., Farmingdale, N.Y.). Residues were resuspended in 200 μl of water, and aliquots were injected into the HPLC column.
HPLC analysis.
NHC and metabolites were separated by reverse-phase HPLC with a Columbus 5-μm-diameter C18 column (Phenomenex, Torrance, Calif.) using a Pro Star model 210 HPLC with manual injection (Varian, Walnut Creek, Calif.). The mobile phase consisted of buffer A (25 mM ammonium acetate with 5 mM TBAP [pH 7.0]) and buffer B (methanol). Elution was performed using a multistage linear gradient of buffer B from 0 to 5% during the first 5 min and then by leveling the buffer to 5% until 10 min at a constant flow of 0.8 ml/min had passed. The elution flow was then increased to 1 ml/min, and the gradient of buffer B was increased from 5 to 15% at 20 min and reached 20% at 35 min; it was kept stable from 35 to 45 min, raised to 30% at 50 min, and finally increased by 5% every 5 min until 60 min had passed. Radioactivity was analyzed with a 500TR Radiometric Flo-One radiochromatography analyzer (Perkin Elmer, Life and Analytical Sciences, Wellesley, Mass.). NHC and the respective metabolites were identified by comparing their chromatographic profiles with those of authentic standards and by enzymatic digestion of whole-cell extracts with alkaline phosphatase.
RESULTS
HPLC analysis of NHC metabolism in liver cells.
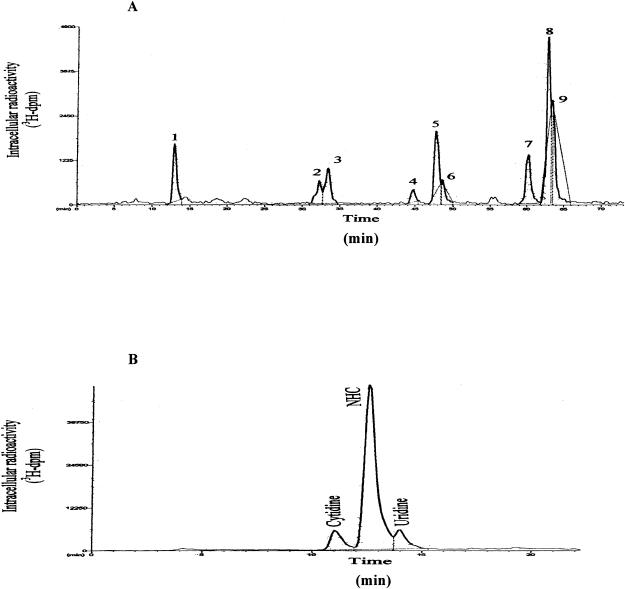
The intracellular metabolism of radiolabeled NHC in primary human hepatocytes, HepG2, Huh-7, and clone A cells was assessed by reverse-phase HPLC, using an ion-pairing method. The HPLC radiochromatogram of clone A cell extracts exposed to [3H]NHC at 4 h is shown in Fig. 1A. Similar HPLC profiles for [3H]NHC in Huh-7 and HepG2 cells and primary human hepatocytes were found (data not shown).
FIG. 1.
HPLC radiochromatograms of intracellular extracts from clone A cells following addition of 10 μM [3H]NHC for 4 h (A) and after digestion with alkaline phosphatase (B) (see Materials and Methods). Metabolites were separated by reverse-phase HPLC with a Columbus 5-μm-diameter C18 column (Phenomenex) using a Pro Star model (Varian) with manual injection. The mobile phase consisted of buffer A (25 mM ammonium acetate with 5 mM TBAP, pH 7.0) and buffer B (methanol). Elution was performed using a multistage linear gradient of buffer B.
The retention times of the parent nucleoside NHC, NHC-MP, NHC-5′-diphosphate (NHC-DP), and NHC-TP are, approximately, 12.5, 32 (peak 2), 47.7 (peak 5), and 62.9 (peak 8) min, respectively. They were initially identified with authentic unlabeled standards. Six additional peaks were eluted in the cells extracted (Table 1; Fig. 1A). Further analysis of these six unknown metabolites was conducted using authentic unlabeled standards, digestion with alkaline phosphatase, or the LC-MS technique.
TABLE 1.
Uptake of 10 μM [3H]NHC (500 dpm/pmol) in liver cells at different time points
| Time (h) | Cell line | Intracellular concn (pmol/106 cells) of the following metabolite (peaks, retention time [min]):
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CMP (peak 1, 14 min) | NHC-MP (peak 2, 32 min) | UMP (peak 3, 33.5 min) | CDP (peak 4, 45 min) | NHC-DP (peak 5, 48 min) | UDP (peak 6, 49 min) | CTP (peak 7, 60 min) | NHC-TP (peak 8, 63 min) | UTP (peak 9, 64 min) | ||
| 1 | Clone A | 13.9 ± 2.1a | 3.4 ± 0.5 | 3.0 ± 0.5 | 3.3 ± 2.1 | 12.3 ± 6.6 | 2.4 ± 0.5 | 5.6 ± 1.7 | 47.6 ± 5.1 | 12.2 |
| Huh-7 | 1.6 ± 0.1 | 16.4 ± 0.1 | 13.8 ± 0.1 | 2.1 ± 0.1 | 9.6 ± 0.1 | 1.3 ± 0.4 | 6.6 | 12.5 ± 4.5 | 57.2 ± 5.3 | |
| HepG2 | 1.9 ± 0.5 | 5.9 ± 2.4 | 20.0 ± 1.5 | 1.6 ± 0.9 | 1.2 ± 0.7 | 3.5 ± 0.5 | 5.2 ± 1.1 | 24.0 ± 5.1 | 28.6 ± 3.2 | |
| Primary hepatocytes | 1.1 ± 0.4 | 3.6 ± 1.0 | 4.9 ± 0.9 | ND | 0.9 | 1.6 ± 0.1 | ND | 1.8 ± 0.3 | 6.8 ± 3.0 | |
| 2 | Clone A | 11.9 ± 2.6 | 4.9 ± 0.1 | 8.3 ± 0.1 | 4.2 ± 1.7 | 14.1 ± 4.4 | 4.9 ± 2.1 | 11.6 ± 0.1 | 64.2 ± 15.4 | 15.3 ± 0.2 |
| Huh-7 | 2.1 ± 0.7 | 31.1 ± 2.7 | 31.6 ± 1.0 | 7.7 ± 3.5 | 19.4 ± 6.8 | 2.3 ± 0.1 | 12.5 ± 3.8 | 22.7 ± 6.1 | 70.6 ± 18.0 | |
| HepG2 | 1.8 ± 0.1 | 9.9 ± 1.4 | 40.6 ± 1.4 | 3.9 ± 0.9 | 3.0 ± 0.1 | 5.1 ± 0.8 | 10.6 ± 2.0 | 50.7 ± 7.5 | 48.3 ± 9.0 | |
| Primary hepatocytes | 1.18 ± 0.16 | 11.6 ± 0.7 | 11.8 ± 2.8 | ND | 2.6 ± 0.9 | 3.7 ± 0.2 | 1.5 ± 0.5 | 3.8 ± 0.1 | 15.7 ± 0.7 | |
| 4 | Clone A | 10.2 ± 0.7 | 6.6 ± 1.1 | 12.1 ± 2.0 | 5.6 ± 0.4 | 14.7 ± 9.2 | ND | 12.6 ± 1.3 | 41.7 ± 11.7 | 7.2 ± 6.1 |
| Huh-7 | 1.7 ± 0.1 | 34.1 ± 0.8 | 52.7 ± 2.1 | 9.0 ± 2.4 | 16.2 ± 6.1 | 5.1 ± 1.4 | 18.7 ± 4.2 | 48.3 ± 14.3 | 78.7 ± 15.3 | |
| HepG2 | 11.6 ± 1.1 | 11.9 ± 0.6 | 44.5 ± 0.8 | ND | 3.4 ± 0.9 | 9.1 ± 0.9 | 10.5 ± 0.8 | 72.5 ± 30.3 | 36.2 ± 5.6 | |
| Primary hepatocytes | 2.8 ± 0.3 | 40.9 ± 9.3 | 45.0 ± 8.2 | 1.2 ± 0.2 | 5.4 ± 1.0 | 21.4 ± 4.5 | 6.0 ± 0.9 | 7.8 ± 0.5 | 33.7 ± 3.8 | |
| 8 | Clone A | 2.1 ± 0.7 | 11.6 ± 2.1 | 40.7 ± 6.1 | 18.2 ± 1.8 | 12.4 ± 8.3 | 17.6 ± 2.1 | 34.8 ± 3.2 | 72.3 ± 24.1 | 36.7 ± 6.2 |
| Huh-7 | 2.3 ± 0.9 | 24.3 ± 0.7 | 51.4 ± 4.0 | 13.1 ± 2.5 | 12.9 ± 2.8 | 9.2 ± 1.6 | 19.9 ± 2.8 | 68.1 ± 3.9 | 60.0 ± 4.7 | |
| HepG2 | 19.5 ± 1.1 | 13.1 ± 3.0 | 32.8 ± 2.1 | 8.8 | 3.4 ± 0.5 | 17.2 ± 0.5 | 7.8 | 120.6 ± 6.5 | 22.1 | |
| 24 | Clone A | ND | 13.1 ± 6.2 | 48.2 ± 7.5 | 8.9 ± 2.8 | 4.0 ± 1.8 | 13.6 ± 4.4 | 33.2 ± 4.7 | 19.3 ± 0.7 | 32.9 ± 5.9 |
| Huh-7 | 1.8 ± 0.3 | 10.7 ± 1.6 | 24.8 ± 2.8 | 21.9 ± 7.3 | 10.0 ± 6.2 | 4.2 ± 1.0 | 14.9 ± 1.1 | 30.0 ± 10.7 | 24.8 ± 7.9 | |
| HepG2 | 28.1 ± 1.6 | 3.9 ± 2.3 | 13.9 ± 1.2 | 1.0 | 2.7 ± 1.1 | 9.0 ± 1.7 | 6.1 ± 0.0 | 23.7 ± 9.8 | 12.4 ± 2.2 | |
| Primary hepatocytes | 4.5 ± 0.7 | 123.5 ± 23.0 | 147.6 ± 33.4 | 4.6 ± 0.9 | 24.9 ± 6.2 | 121.5 ± 26.0 | 16.3 ± 3.7 | 3.4 ± 0.4 | 106.8 ± 20.1 | |
Values are means ± standard deviations from three independent experiments. ND, not detected (limit of detection, ∼0.05 pmol/106 cells).
Characterization of unknown metabolites by treatment with alkaline phosphatase.
To characterize the unknown metabolites, in cell extracts, NHC-5′-phosphate metabolites were incubated with alkaline phosphatase. This treatment resulted in the digestion of almost all peaks except the three peaks with retention times of 11.0, 12.5, and 14.0 min, which indicated that no terminal phosphate group was present (Fig. 1B). The three peaks obtained from the alkaline phosphatase digestion were identified as cytidine, NHC, and uridine by comparison of the retention times of authentic standards.
LC-MS-MS analysis.
The retention times of the standard compounds were as follows: 32.6 min (NHC-TP), 32.8 min (CTP), and 33.6 min (UTP). The maximum wavelengths at a pH of 7 for NHC-TP, CTP, and UTP were 236.8, 272.1, and 262.7 nm, respectively.
Using LC-MS-MS technology, the following fragmentation schemes were obtained for the following triphosphates: for CTP, m/z 481.6 to 402.1, 383.7, 158.8 with CID 30 eV; for UTP, m/z 482.6 to 402.3, 385.3, 176.9, 158.8 with CID 30 eV; and for NHC-TP, m/z 497.5 to 399.9, 158.7 with CID 30 eV.
The same LC-MS-MS conditions were applied to the Huh-7 cell extracts. Three injections of the same Huh-7 sample were necessary to obtain the fragmentation schemes of the two unknown metabolites, peaks 7 (retention time, ∼60 min) and 9 (retention time, ∼64 min) (Table 1; Fig. 1A).
After the characterization of cytidine and uridine as principal metabolites of NHC (Fig. 1B), LC-MS-MS was used for the identification of peaks 7 and 9 from the cell extracts. After the conditions were set, cold cell extract samples were injected and the peaks were identified as CTP (peak 7), NHC-TP (peak 8), and UTP (peak 9). Since the alkaline phosphatase digestion indicated the presence of cytidine and uridine, the unknown peaks 1, 3, 4, and 6 were characterized with authentic unlabeled standards of MP and DP of cytidine and uridine based on their masses, fragmentation patterns, and metabolism patterns (Table 1).
These results illustrate that NHC is metabolized in the cytoplasm of the liver cells to corresponding NHC-MP, NHC-DP, and NHC-TP; in addition, the different metabolites of cytidine and uridine MP, DP, and TP were also detected.
Uptake of NHC in liver cells.
Uptake studies were conducted with different cell lines in triplicate to determine the levels of all intracellular metabolites of radiolabeled NHC. Primary human hepatocytes, clone A, Huh-7, and HepG2 cell lines were incubated with 10 μM [3H]NHC (500 dpm/pmol) for different time points at 37°C. For discussion purposes, the 4-h time point instead of the 8-h time point was chosen when most of the nucleoside triphosphate metabolites reached the maximum concentration in liver cell lines (Table 1). Metabolites corresponding to NHC-TP reached intracellular concentrations of 41.7 ± 11.7, 48.3 ± 14.3, 72.4 ± 30.3, and 7.8 ± 0.5 pmol/106 cells (means ± standard deviations) at 4 h of incubation in clone A cells, Huh-7 cells, HepG2 cells, and primary human hepatocytes, respectively.
CTP and UTP (derived from NHC) achieved concentrations of 12.6 ± 1.3 and 7.2 ± 6.1 pmol/106 cells in clone A cells, 18.7 ± 4.2 and 78.7 ± 15.3 pmol/106 cells in Huh-7 cells, and 10.5 ± 0.8 and 36.2 ± 5.6 pmol/106 cells in HepG2 cells, respectively, at 4 h. In primary human hepatocytes, CTP and UTP reached the maximum concentrations of 6.0 ± 0.9 and 33.7 ± 3.8 pmol/106 cells, respectively, by 4 h.
Determination of intracellular NHC-TP t1/2s.
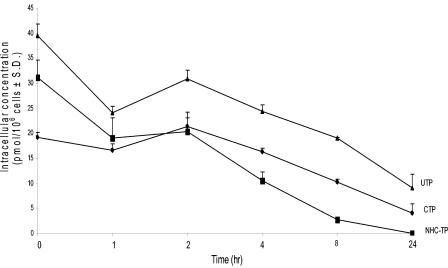
The t1/2 of NHC-TP was measured in Huh-7 cells (Fig. 2) and was at least 3 h, with the concentration reaching to 2.7 ± 0.0 pmol/106 cells at 8 h after removal of the respective parent drug. The t1/2s of the other two radioactive nucleotide triphosphates, CTP and UTP, derived from radiolabeled NHC incubation, were found to be 10.4 ± 3.3 and 13.2 ± 3.5 h, respectively.
FIG. 2.
Decay of [3H]NHC-TP metabolites after incubation of Huh-7 cells for 24 h (10 μM). TP t1/2 values for cytidine, NHC, and uridine were 10.4 + 3.3, 3.0 + 1.3, and 13.2 + 3.5 h, respectively (n = 3). The exponential rates of decline (κ) for NHC-TP, UTP, and CTP were calculated as the slope of the natural logarithm of the respective concentrations versus time by using the terminal linear portion of the curve. The t1/2 of decay was determined to be −0.693/κ.
Stability studies of NHC in human and monkey blood.
The stability of NHC in monkey and human blood was analyzed at different time points over a period of 0 to 24 h at 37°C. In monkey blood, cytidine and uridine concentrations increased with time, reaching 10.2 and 64.2%, respectively, after 24 h. In human blood, the values were 7.7 and 52.4%, respectively (Table 2). NHC was not stable in either monkey or human blood, since at 24 h, NHC levels were 25.6% in monkey blood and 39.9% in human blood, although NHC was markedly more stable in human whole blood than in monkey whole blood at 4 h (92% versus 49.2% of NHC was unchanged) (Table 2). The t1/2s of NHC, cytidine, and uridine in monkey blood were 3.9, 0.9, and 4.9 h, respectively. In human blood, NHC and uridine had t1/2s of 17.9 and 6.9 h, respectively. The t1/2 of cytidine in human whole blood was not quantifiable, since the nucleoside levels were minimal up to 24 h after exposure to NHC.
TABLE 2.
Stability study of 10 μM [3H]NHC (1,000 dpm/pmol) in monkey and human whole blood
| Time (h) | % Nucleoside in:
|
|||||
|---|---|---|---|---|---|---|
| Monkey blood
|
Human blood
|
|||||
| Cytidine | NHC | Uridine | Cytidine | NHC | Uridine | |
| 0 | 0 | 100.0 | 0 | 0 | 100 | 0 |
| 0.08 | 0 | 99.0 | 1.0 | |||
| 0.16 | 0.3 | 91.7 | 8.02 | |||
| 1 | 0.5 | 85.0 | 14.5 | 0 | 95.3 | 4.7 |
| 2 | 0.0 | 65.2 | 34.9 | |||
| 4 | 0.6 | 49.2 | 50.2 | 0 | 92.0 | 8.0 |
| 24 | 10.2 | 25.6 | 64.2 | 7.7 | 39.9 | 52.4 |
| t1/2 | 0.90 | 3.89 | 4.93 | 17.90 | 6.87 | |
DISCUSSION
NHC is a potent and selective antiviral agent in the HCV replicon system and against bovine viral diarrhea virus in culture (19). NHC did not exhibit either cellular or mitochondrial toxicity in liver cells (19). In this paper, we focused on the metabolism of this base-modified ribonucleoside analogue in liver cells. Incubation of [3H]NHC in the four liver cell systems led to the formation of NHC-MP, -DP, and -TP, along with six unknown metabolites. Digestion of cell extracts incubated with [3H]NHC with alkaline phosphatase led to three peaks, two of which were unknown for metabolites that were characterized as cytidine, uridine, and NHC by using authentic standards. LC-MS-MS analysis of the pure authentic standards confirmed the structure of the metabolites based on the retention times, the UV spectra, and the fragmentation schemes of the three nucleotides. Evaluation of the Huh-7 sample after 24 h of incubation with NHC showed the presence of UTP and CTP as metabolites. Alkaline phosphatase digestion and the LC-MS-MS technique further confirmed the presence of cytidine, uridine, and the MPs, DPs, and TPs of cytidine and uridine after comparison to authentic standards.
Uptake studies of NHC indicated rapid phosphorylation and high NHC-TP, CTP, and UTP levels in clone A, Huh-7, HepG2 cells, and primary human hepatocytes after 4 h of incubation. In clone A cells, NHC-TP was the predominant metabolite up to 8 h. At 8 h, CTP and UTP reached similar levels, and by 24 h, UMP, UTP, and CTP were the predominant metabolites (Table 1). Although the Huh-7 cell line is the parental cell line of clone A cells, the maximum UTP intracellular concentration was attained earlier at 2 h. From 8 to 24 h, the intracellular concentrations of NHC-TP and UTP were similar. In the HepG2 cell line, NHC-TP was the predominant metabolite from 4 to 24 h, but at early times, UTP levels were higher. Primary human hepatocytes incubated with tritiated NHC phosphorylated this nucleoside rapidly, but up to 24 h, one of the predominant metabolites was UTP, reaching levels of 106.8 ± 20.1 pmol/106 cells at 24 h (Table 1).
Recently, Stuyver and colleagues (19) determined that the EC90 of NHC in the replicon system was 5 μM. In our uptake studies, at 1 h, NHC-TP, the active metabolite, was formed in Huh-7 and clone A cells at levels equivalent to 2 to 10 times higher than the EC90s in the replicon system. However, as radiolabeled NHC was incubated for up to 8 h, the level of NHC-TP increased in all the liver cell lines described in this paper, including in primary human hepatocytes. For example, in primary human hepatocytes, NHC-TP achieved levels of 7.8 pmol/106 cells, which is higher than the in vitro EC90 in the replicon system. However, it should be noted that these cells were bought in plates and were near confluence when they were used for these uptake studies. This may explain the relatively lower levels of NHC-TP formed in primary human hepatocytes than in rapidly dividing Huh-7, clone A, and HepG2 cells (Table 1).
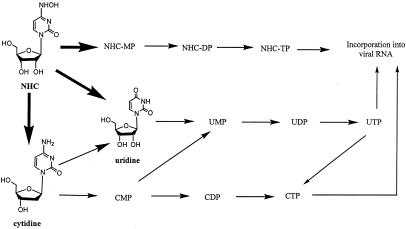
The higher intracellular levels of UTP are explained in Fig. 3, which shows how radioactive NHC was transformed to cytidine and uridine by different pathways. Cytidine was also deaminated to uridine, which was then phosphorylated to its MP, DP, and TP forms. In the four liver cell systems, CTP showed the lowest intracellular concentration compared to those of NHC-TP and UTP, probably because cytidine is not only phosphorylated, but also deaminated to uridine.
FIG. 3.
Proposed metabolism for NHC in liver cells.
The intracellular decay of NHC-TP demonstrated a short t1/2 of 3.0 ± 1.3 h compared to the t1/2s of CTP and UTP derived from NHC incubation (10.4 ± 3.3 and 13.2 ± 3.5 h, respectively) in Huh-7 cells. This shorter NHC-TP t1/2 probably reflects a higher affinity of NHC for catalytic enzymes, presumably cytidine deaminase and an unknown oxidative enzyme. Furthermore, the t1/2 of NHC-TP is relatively short (3.00 ± 1.34 h) compared to those of UTP and CTP produced from NHC-TP or its metabolites.
Stability studies of radiolabeled NHC in monkey and human whole blood suggested that this compound might not persist long enough in plasma at a physiological pH and temperature to deliver significant antiviral levels of NHC into HCV-infected liver cells. The predominant and first metabolite found in monkey and human blood was uridine. Although detectable or moderate concentrations of cytidine, a product of NHC reduction, were found, uridine was the most prevalent metabolite. The high percentage of uridine in monkey and human blood up to 24 h and its rapid detection suggest that NHC can be oxidized and reduced by different pathways.
The high levels of uridine and its metabolites can be explained by analyzing the data from the stability study of monkey and human blood and the uptake of NHC in human liver cells. The data suggest that NHC is converted to uridine and also to cytidine, which is then deaminated to uridine. Both pathways contribute to the intracellular concentrations of uridine and its metabolites.
The deamination mechanism of nucleosides in different species has been described previously (3, 10). Those reports are consistent with our results where deamination in monkey blood is higher than in human blood, in which NHC is more stable, with a t1/2 of 17.9 h, compared to 3.9 h in monkey blood.
The finding of high levels of these metabolites in cells exposed to NHC could have implications, since earlier studies suggest that uridine and cytidine may prevent the anti-HCV activity of NHC (19). In addition, production of these metabolites may create feedback inhibition, reducing the absolute levels of NHC-TP.
A proposed metabolic pathway is shown in Fig. 3. Based on kinetic studies, the first metabolite formed after the incubation of NHC in monkey and human blood was uridine, and the second was cytidine. Radiolabeled NHC, cytidine, and uridine in monkey plasma had intracellular t1/2s of 3.9, 0.9, and 4.9 h, respectively. In human plasma, NHC and uridine had t1/2s of 17.9 and 6.9 h, respectively. The short intracellular t1/2 of NHC-TP supports the proposed deamination and oxidation of NHC (Fig. 3), since the incubation of radiolabeled NHC led to the formation of cytidine and uridine MPs, DPs, and TPs. Early studies with NHC in growing cells of Salmonella enterica serovar Typhimurium support the proposed metabolic pathway (14, 15). It was demonstrated that a bacterial enzyme system was capable of transforming NHC to cytidine by direct deamination.
As demonstrated in this paper, incubation of [3H]NHC in various liver cells leads to its phosphorylation and to the cytidine and uridine MPs, DPs, and TPs. The levels of NHC-TP in both the clone A cells used in the replicon system and primary human hepatocytes were in excess of the EC90 for inhibition of HCV at 4 h after cell exposure to radioactive NHC.
Taken together, these results suggest that similar metabolisms may occur in animals and humans. Thus, approaches to prevent the oxidation or reduction of NHC should be a priority for advancing this nucleoside to the clinic. Based on these studies, it is also apparent that N4-hydroxypyrimidine nucleosides could be used as prodrugs for sugar-modified cytosine and uracil nucleoside analogues. Such prodrug approaches are currently being developed in our laboratories for a variety of new antiviral agents.
Acknowledgments
R.F.S. is supported in part by the Department of Veterans Affairs and NIH grant 2P30-AI-50409. B.I.H.-S. is supported by a supplement to NIH grant R37-AI-41980. L.S. is supported by NIH grant R34-AI-52686.
R.F.S. is the principal founder of and a scientific consultant to Pharmasset Inc., and his particulars have been reviewed by Emory University's Conflict of Interest Committee. His group received no funding from Pharmasset or any other company to perform this work.
REFERENCES
- 1.Alter, M. J. 1997. Epidemiology of hepatitis C. Hepatology 26(3 Suppl. 1):62S-65S. [DOI] [PubMed] [Google Scholar]
- 2.Becher, F., D. Schlemmer, A. Pruvost, M. C. Nevers, C. Goujard, S. Jorajuria, C. Guerreiro, T. Brossette, L. Lebeau, C. Creminon, J. Grassi, and H. Benech. 2002. Development of a direct assay for measuring intracellular AZT triphosphate in human peripheral blood mononuclear cells. Anal. Chem. 74:4220-4227. [DOI] [PubMed] [Google Scholar]
- 3.Camiener, G. W., and C. G. Smith. 1965. Studies of the enzymatic deamination of cytosine arabinose. I. Enzyme distribution and species specificity. Biochem. Pharmacol. 14:1405-1416. [DOI] [PubMed] [Google Scholar]
- 4.Decoster, L. A., E. Cottin, X. Chen, F. Lejeune, R. O. Mirimanoff, J. Biollaz, and P. A. Coucke. 1999. Simultaneous determination of deoxyribonucleoside in the presence of ribonucleoside triphosphate in human carcinoma cells by high-performance liquid chromatography. Anal. Biochem. 270:59-68. [DOI] [PubMed] [Google Scholar]
- 5.Di Pierro, D., B. Tavazzi, C. F. Perno, M. Bartolini, E. Balestra, R. Calio, B. Giardina, and G. Lazzarino. 1995. An ion-pairing high-performance liquid chromatographic method for the direct simultaneous determination of nucleotides, deoxynucleotides, nicotinic coenzymes, oxypurines, nucleosides, and bases in perchloric acid cell extracts. Anal. Biochem. 231:407-412. [DOI] [PubMed] [Google Scholar]
- 6.Falck-Ytter, Y., H. Kale, K. D. Mullen, S. A. Sarbah, L. Sorescu, and J. McCullough. 2002. Surprisingly small effect of antiviral treatment in patients with hepatitis C. Ann. Intern. Med. 136:288-292. [DOI] [PubMed] [Google Scholar]
- 7.Fung, E. N., Z. Cai, T. C. Burnette, and A. K. Sinhababu. 2000. Simultaneous determination of nucleoside and nucleotide analogs in human extracts at nanomolar levels by using ion-pairing HPLC-MS-MS, poster MPI-290. .In Proceedings of the 48th ASMS Conference on Mass Spectrometry, Long Beach, Calif.
- 8.Fung, E. N., Z. Cai, T. C. Burnette, and A. K. Sinhababu. 2001. Simultaneous determination of Ziagen and its phosphorylated metabolites by ion-pairing high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 754:285-295. [DOI] [PubMed] [Google Scholar]
- 9.Greenwood, R. C., and D. R. Gentry. 2002. The effect of antibiotic treatment on the intracellular nucleotide pools of Staphylococcus aureus. FEMS Microbiol. Lett. 208:203-206. [DOI] [PubMed] [Google Scholar]
- 10.Kelley, J. A., C. L. Litterst, J. S. Roth, D. T. Vistica, D. G. Poplack, D. A. Cooney, M. Nadkarni, F. M. Balis, S. Broder, and D. G. Johns. 1987. The disposition and metabolism of 2′,3′-dideoxycytidine, an in vitro inhibitor of human T-lymphotrophic virus type III infectivity, in mice and monkeys. Drug Metab. Dispos. 15:595-601. [PubMed] [Google Scholar]
- 11.Liu, X., and R. F. Schinazi. 2003. Molecular and serum epidemiology of HBV and HCV infection and the impact of antiviral agents in China, p. 15-33. In R. F. Schinazi, J.-P. Sommadossi, and C. M. Rice (ed.), Frontiers in viral hepatitis. Elsevier B. V., Amsterdam, The Netherlands.
- 12.McHutchison, J. G., S. C. Gordon, E. R. Schiff, M. L. Shiffman, W. M. Lee, V. K. Rustgi, Z. D. Goodman, M.-H. Ling, S. Cort, J. K. Albrecht, et al. 1998. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N. Engl. J. Med. 339:1485-1492. [DOI] [PubMed] [Google Scholar]
- 13.Piliero, P. J., and J. J. Faragon. 2002. Hepatitis B virus and HIV coinfection. AIDS Read. 12:448-451. [PubMed] [Google Scholar]
- 14.Popowska, E., and C. Janion. 1975. The metabolism of N4-hydroxycytidine—a mutagen for Salmonella typhimurium. Nucleic Acids Res. 2:1143-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popowska, E., and C. Janion. 1977. The N4-hydroxycytidine reduction system in toluenized cells of Salmonella typhimurium. Acta Biochim. Pol. 24:197-205. [PubMed] [Google Scholar]
- 16.Poynard, T., P. Marcellin, S. S. Lee, C. Niederau, G. S. Minuk, G. Ideo, V. Bain, J. Heathcote, S. Zeuzem, C. Trepo, J. Albrecht, et al. 1998. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet 352:1426-1432. [DOI] [PubMed] [Google Scholar]
- 17.Pruvost, A., F. Becher, P. Bardouille, C. Guerrero, C. Creminon, J. F. Delfraissy, C. Goujard, J. Grassi, and H. Benech. 2001. Direct determination of phosphorylated intracellular anabolites of stavudine (d4T) by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 15:1401-1408. [DOI] [PubMed] [Google Scholar]
- 18.Raab, M., H. Daxecker, A. Karimi, S. Markovic, M. Cichna, P. Markl, and M. M. Muller. 2001. In vitro effects of mycophenolic acid on the nucleotide pool and on the expression of adhesion molecules of human umbilical vein endothelial cells. Clin. Chim. Acta 310:89-98. [DOI] [PubMed] [Google Scholar]
- 19.Stuyver, L. J., T. Whitaker, T. R. McBrayer, B. I. Hernandez-Santiago, S. Lostia, P. M. Tharnish, M. Ramesh, C. K. Chu, R. Jordan, J. Shi, S. Rachakonda, K. A. Watanabe, M. J. Otto, and R. F. Schinazi. 2003. Ribonucleoside analogue that blocks replication of bovine viral diarrhea and hepatitis C viruses in culture. Antimicrob. Agents Chemother. 47:244-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. 1997. Hepatitis C: global prevalence. Wkly. Epidemiol. Rec. 72:341-344. [PubMed] [Google Scholar]