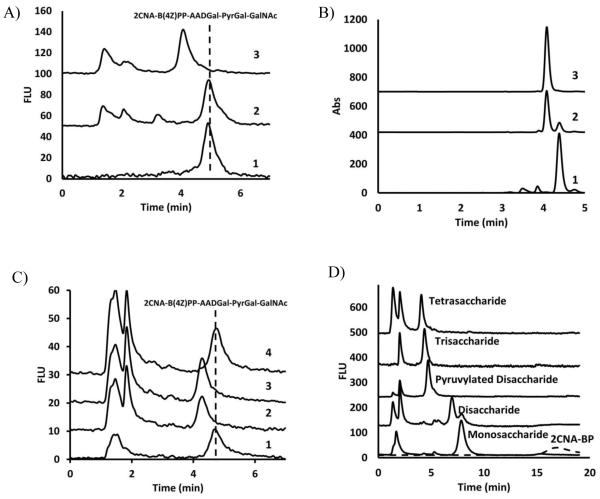
Figure 5. WcfN catalyzes the transfer of galactofuranose completing tetrasaccharide formation.
A) 2CNA-B(4Z)PP-AADGal-PyrGal-GalNAc was mixed with 1) No additional enzyme or sugar donor, 2) UDP-Galactopyranose and WcfN and 3) UDP-Galf and WcfN confirming the role of WcfN in the final step of repeat unit biosynthesis. B) HPLC analysis of 1) Pure UDP-Galf, 2) WcfM reaction with UDP-Galp, 3) Pure UDP-Galp. C) HPLC analysis of reactions containing 2CNA-B(4Z)PP-AADGal-PyrGal-GalNAc with 1) No enzyme or sugar donor, 2) UDP-Galf and WcfN, 3) UDP-Galp, WcfM and WcfN, 4) UDP-Galp and WcfN. D) Starting with bactoprenyl phosphate, enzymes were sequentially added leading to the formation of each intermediate product in the pathway and the single pot formation of the tetrasaccharide. HPLC with isoprenoid linked materials was performed with a mobile phase of 32% 1-Propanol in 100 mM ammonium bicarbonate. HPLC conditions for UDP-linked sugars used a mobile phase of 1.5% acetonitrile in 50mM triethylammonium acetate buffer (pH 6.8).