Abstract
Purpose
Previous studies have demonstrated the immunogenicity and safety of the co-administration of the trivalent inactivated influenza vaccine (IIV3) with the polysaccharide pneumococcal vaccine (PPV) or pneumococcal conjugate vaccine (PCV). However, there is no direct comparison study that evaluates the immunogenicity and safety of IIV3 given concomitantly with PCV13 or PPV23 in the elderly.
Materials and Methods
During the 2012-2013 influenza vaccination period, 224 healthy elderly volunteers aged 65 years and older randomly received IIV3 given concomitantly with either PCV13 (PCV13+IIV3) or PPV23 (PPV23+IIV3) in a 1:1 ratio. Serum hemagglutination-inhibiting antibodies for IIV3 were measured at the time of vaccination and 1 month after vaccination. Adverse events were recorded prospectively in a clinical diary during a 7-day period.
Results
A total of 220 participants blood samples for analysis of immunogenicity and kept a clinical diary for safety analysis (PCV13+IIV3, n=110; PPV23+IIV3, n=110). One month after vaccination, both groups satisfied the Committee for Medical Products for Human Use criteria for A/H1N1, A/H3N2 and B strains, showing comparable seroprotection rates, seroconversion rates and geometric mean titer fold. The assessments of immunogenicity were similar in both groups. The most common local and systemic reactions were pain at the injection site and generalized myalgia. They were generally mild or moderate in intensity. The adverse events were not statistically different between the two groups.
Conclusion
PCV13+IIV3 and PPV23+IIV3 demonstrated similar immunogenicity and safety in the elderly.
Keywords: Pneumococcus, Influenza, Vaccine, Immunogenicity, Safety
Introduction
The concurrent infection of influenza and pneumococcus is commonly observed during the circulation of influenza, and both infection causes the leading mortality in the elderly [1,2]. Vaccination against both of these diseases is important to prevent infections and their complications [3,4,5,6]. Delivering both vaccines together not only increases the vaccination rate but also decreases mortality and hospitalization rates in the elderly [7,8,9]. However, only 20%-40% of older adults receive pneumococcal vaccine in most countries [10]. In the Republic of Korea, concomitant administration of the influenza vaccine and the pneumococcal vaccine has been recommended to improve the vaccination rates during the influenza vaccination period [11].
At the time of this study, a 13-valent pneumococcal conjugate vaccine (PCV13) and a 23-valent polysaccharide pneumococcal vaccine (PPV23) were introduced for adults in Korea. However, there was no preferential recommendation for use of PCV13 or PPV23 targeting the elderly. The selection of pneumococcal vaccine type was totally depended on vaccinee's opinion.
Comparability of immunogenicity and safety of trivalent inactivated influenza vaccine (IIV3) co-administered with PPV23 or PCV13 has been previously demonstrated [12,13]. However, there is no direct comparison study that evaluates the immunogenicity and safety of IIV3 given concomitantly with PCV13 or IIV3 given with PPV23 in the elderly. We performed a multicenter, randomized, comparative study in people aged 65 years or older who had not previously received a pneumococcal vaccine. This study will clarify the relative immunogenicity and tolerability of IIV3 when simultaneously administered with PCV13 or PPV23.
Materials and Methods
Study participants
The multicenter, randomized, comparative study was conducted during the 2012-2013 influenza vaccination period. (Clinical Trial Number: NCT0258047) Participants were enrolled at two centers during the last week of October 2012. Eligible participants were healthy men and women aged 65 years or older who were naïve to the 2012/2013 season influenza vaccine and any pneumococcal vaccine. Exclusion criteria were as follows: known egg allergy, febrile illness on the day of vaccination, any other vaccination within the previous 30 days, immunodeficiency or suppression, treatment with immunoglobulins during the past 3 months, a history of Streptococcal pneumoniae infection within the previous 5 years, and any conditions which might interfere with the study results.
Vaccines and administration
PCV13 (Prevenar 13, Wyeth Pharmaceuticals Inc., Marietta, GA, USA) contained polysaccharides of pneumococcal serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19F, 19A, and 23F conjugated to a nontoxic mutant form of diphtheria toxin cross-reactive material 197. Each 0.5-mL vial contained 2.2 µg of each serotype, except type 6B, which was included at 4.4 µg.
PPV23 (Pneumovax 23, Merck & Company, Inc., Kenilworth, NJ, USA) consisted of a purified capsular polysaccharide from 12 of the serotypes included in PCV13 (all except 6A), as well as 11 additional serotypes (2, 8, 9N, 10A, 11A, 12F, 15B, 17F, 20, 22F, and 33F). The vaccine was formulated to contain 25 µg of each of the 23 purified polysaccharide serotypes per 0.5 mL vial.
IIV3 (GCflu, Green Cross Corp., Yongin, Korea) was formulated according to the World Health Organization recommendations for the 2012/2013 (northern hemisphere) flu season and contained 15 µg hemagglutinin for each of the three influenza strains per 0.5 mL vial: an A/California/7/2009 (H1N1)-like virus, an A/Victoria/361/2011 (H3N2)-like virus, and a B/Hubei-Wujiagang/158/2009-like virus.
All eligible participants randomly received IIV3 given concomitantly with either PCV13 (PCV13+IIV3) or PPV23 (PPV23+IIV3) in a 1:1 ratio using an excel-based randomization program. The influenza vaccine was administered to the right deltoid and the pneumococcal vaccines were administered to the left deltoid.
Immunogenicity assessments
We obtained blood samples from each patient before vaccination and then at 28±7 days after vaccination. Hemagglutination-inhibiting (HI) antibodies were measured by a standard microtiter assay using washed turkey erythrocytes [14,15]. The serum HI antibodies were determined in the range of 1:10 to 1:5,120. A titer of <1:10 was assigned a value of 1:5, while titers above 1:5,120 were assigned a value of 1:5,120.
Geometric mean titer (GMT), seroprotection rate (proportion of subjects with a post-vaccination titer ≥1:40), seroconversion rate (proportion of subjects with ≥fourfold increase in titer from pre-vaccination titer or a post-vaccination ≥1:40 if the pre-vaccination titer was <1:40), and GMT fold (GMT ratio of the post-vaccination titer to pre-vaccination titer) were measured. The criteria of the Committee for Medical Products for Human Use (CHMP) was applied to evaluate immunogenicity; seroprotection rate >60%, seroconversion rate >30%, and GMT fold >2.
Safety assessments
Any solicited local or systemic reactions to both vaccines were monitored using a clinical diary for seven days after vaccination. Participants were asked to record pain, tenderness, and redness diameter at both injection sites and the severity of systemic symptoms such as headache, malaise, chills, muscle aches, arthralgia, and any other adverse events, according to the Food and Drug Administration Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials [16].
Statistical analysis
To estimate local and systemic reactions, the proportions of subjects experiencing reactions were calculated based on the frequency and severity from their submitted diary. The 95% confidence interval was calculated using the Student's t-distribution, assuming a normal distribution of the log10 titer. Two-tailed Fisher exact tests were used to compare both groups. All reported p-values are two-sided, and values of 0.05 or less are considered statistically significant. Data analysis was performed using SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA).
Results
Study subjects
A total of 224 participants were enrolled in the study (PCV13+ IIV3, n=112; PPV23+IIV3, n=112). Of the 224 subjects, 4 participants withdrew consents after vaccination due to personal reasons. Finally, blood samples for analysis of immunogenicity and clinical diaries for safety analysis were available from 220 participants (PCV13+IIV3, n=110; PPV23+IIV3, n=110). No gender difference was seen between two groups (male 46.2% vs. 45.5%, p=0.413). The median ages of subjects were 72 and 73 years, respectively, and were not also statistically different.
Immunogenicity response to IIV3
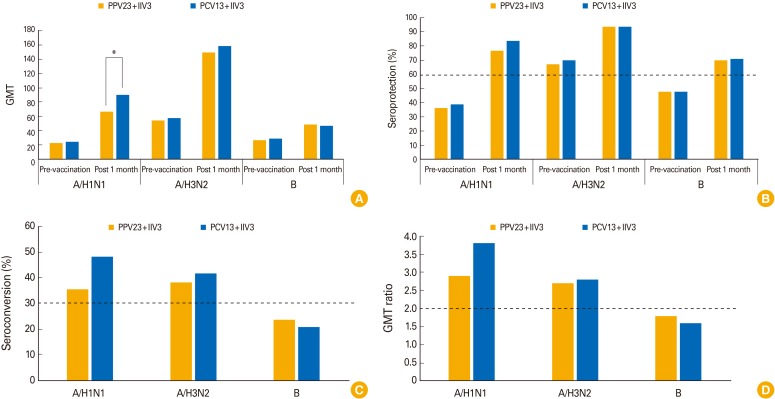
The immunogenicity data for all three influenza virus strains are summarized in Table 1 and Fig. 1. Pre-vaccination GMTs for each strain were similar between the two groups (A/H1N1, p=0.603; A/H3N2, p=0.737; B, p=0.555). One month after vaccination, the GMTs had increased significantly in both vaccine groups for all three strains. The changes in GMTs for A/H3N2 and B were not different. However, the GMTs for A/H1N1 were significantly higher in PCV13+IIV3 than in PPV23+IIV3 (p=0.026). Both groups satisfied all three CHMP criteria for A/H1N1 and A/H3N2 strains, showing comparable seroprotection and seroconversion rates. As for the influenza B strain, neither group showed a sufficient seroconversion rate or increase in GMT. However, both groups satisfied the seroprotection rate criteria. Overall, the assessments of all three criteria were similar in both groups (seroprotection rate, p>0.990; seroconversion rate, p=0.746; GMT fold, p=0.588).
Table 1. Antibody responses as measured with the hemagglutination-inhibition assay according to vaccine group.
| A/H1N1 | A/H3N2 | B | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PPV23 (n = 110) | PCV13 (n = 110) | p-value | PPV23 (n = 110) | PCV13 (n = 110) | p-value | PPV23 (n = 110) | PCV13 (n = 110) | p-value | |
| GMT (95% CI) | |||||||||
| At pre-vaccination | 22.3 (17.5–27.9) | 24.3 (19.4–30.3) | 0.603 | 54.1 (43.1–68.3) | 57.3 (45.1–72.8) | 0.737 | 26.6 (22.3–31.7) | 28.8 (23.4–35.0) | 0.555 |
| At post 1 moa) | 66.4 (55.1–80.1) | 89.9(74.5–108.4) | 0.026 | 149.5 (125.5–178.1) | 158.7 (133.2–189.1) | 0.634 | 48.7 (41.3–57.4) | 46.8 (39.7–55.1) | 0.735 |
| Seroprotection rate (95% CI, %) | |||||||||
| At pre-vaccination | 36.3 (27.4–45.1) | 38.9 (30.1–47.8) | 0.784 | 67.3 (58.4–76.1) | 69.9 (61.1–77.9) | 0.775 | 47.8 (38.1–56.6) | 47.8 (38.1–56.6) | > 0.990 |
| At post 1 mo | 76.4 (67.3–84.5) | 83.6 (77.3–90.0) | 0.238 | 93.6 (88.2–98.2) | 93.6 (88.2–98.2) | 1.000 | 70.0 (60.9–78.2) | 70.9 (62.7–79.1) | > 0.990 |
| Seroconversion rate (95% CI, %) | |||||||||
| At post 1 mo | 35.5 (26.4–44.5) | 48.2 (38.2–58.2) | 0.075 | 38.2 (29.1–48.2) | 41.8 (31.8–51.8) | 0.680 | 23.6 (16.4–32.7) | 20.9 (13.6–28.2) | 0.746 |
| GMT fold (95% CI) | |||||||||
| At post 1 mo | 2.9 (2.3–3.6) | 3.8 (3.0–4.7) | 0.104 | 2.7 (2.2–3.4) | 2.8 (2.3–3.5) | 0.835 | 1.8 (1.5–2.2) | 1.6 (1.4–2.0) | 0.588 |
PPV23, 23-valent polysaccharide pneumococcal vaccine; PCV13, 13-valent pneumococcal conjugate vaccine; GMT, geometric mean titer; CI, confidence interval.
a)Post-vaccination GMT was adjusted according to pre-vaccination titer.
Fig. 1. Changes of geometric mean titer (GMT), seroprotection rate, seroconversion rate, and GMT fold after vaccination. Serum samples collected from elderly volunteers vaccinated with trivalent inactivated influenza vaccine (IIV3) simultaneously with either 13-valent pneumococcal conjugate vaccine (PCV13) or 23-valent polysaccharide pneumococcal vaccine (PPV23) before vaccination and 1 month after vaccination were tested by hemagglutination inhibition assay. The GMT (A), the seroprotection rate (proportion of subjects with a post-vaccination titer ≥1:40) (B), seroconversion rate (proportion of subjects with ≥4-fold increase in titer from pre-vaccination titer or a post-vaccination ≥1:40 if the pre-vaccination titer was <1:40) (C), and GMT fold (GMT ratio of the post-vaccination titer to pre-vaccination titer) (D) were measured. The dotted lines indicate the criteria of the Committee for Medical Products for Human Use (CHMP). *Significantly different between two groups.
Safety
The overall incidence of local reaction is shown in Tables 2 and 3. The most common local reaction was pain at the injection site, which was reported by 24.1% (53 out of total 220 participants) at the pneumococcal vaccination sites and 1.8% (4 out of total 220 participants) at the influenza vaccination sites. Other local reactions were tenderness, redness, and swelling, occurring in sequence more commonly at the pneumococcal vaccination site than the influenza vaccination site (p<0.001). Most pain reactions were reported as mild in severity. An overall systemic reaction occurred in 9.0% of patients in the PCV13+IIV3 group and in 6.4% of patients in the PPV23+IIV3 group (p=0.572). The systemic reactions reported in both groups were generally mild or moderate in intensity. The differences in each reaction were statistically comparable between the two vaccine groups. Unsolicited signs and symptoms were not reported in any of the participants.
Table 2. Local adverse effects within 7 days.
| Local reaction | Pneumococcal vaccination site (n = 220) | Influenza vaccination site (n = 220) | ||||
|---|---|---|---|---|---|---|
| PPV23 (n = 110) | PCV13 (n = 110) | p-value | With PPV23 (n = 110) | With PCV13 (n = 110) | p-value | |
| Pain | 0.888 | 0.372 | ||||
| None | 85 (77.3) | 82 (74.5) | 109 (99.1) | 107 (97.3) | ||
| Mild | 23 (20.9) | 26 (23.6) | 1 (0.9) | 3 (2.7) | ||
| Moderate | 2 (1.8) | 2 (1.8) | 0 (0) | 0 (0) | ||
| Severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Tenderness | 0.827 | > 0.990 | ||||
| None | 89 (80.9) | 87 (79.1) | 109 (99.1) | 108 (98.2) | ||
| Mild | 20 (18.2) | 21 (19.1) | 1 (0.9) | 2 (1.8) | ||
| Moderate | 1 (0.9) | 2 (1.8) | 0 (0) | 0 (0) | ||
| Severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Redness diameter (mm) | 0.613 | > 0.990 | ||||
| 0 | 109 (96.5) | 105 (94.6) | 110 (0) | 110 (0) | ||
| 1-4 | 10 (9.1) | 8 (7.3) | 0 (0) | 0 (0) | ||
| ≥ 5 | 1 (0.9) | 0 (0) | 0 (0) | 0 (0) | ||
| Swelling diameter (mm) | 0.192 | 0.368 | ||||
| 0 | 109 (96.5) | 108 (97.3) | 109 (99.1) | 109 (99.1) | ||
| 1-4 | 6 (5.5) | 2 (1.8) | 1 (0.9) | 0 (0) | ||
| ≥ 5 | 0 (0) | 0 (0) | 0 (0) | 1 (0.9) | ||
Values are presented as number (%).
PPV23, 23-valent polysaccharide pneumococcal vaccine; PCV13, 13-valent pneumococcal conjugate vaccine.
Table 3. Systemic adverse effects within 7 days.
| Systemic reaction | PPV23 + IIV3 (n = 110) | PCV13 + IIV3 (n = 110) | p-value |
|---|---|---|---|
| Fever, temperature ≥ 38℃ | 0 (0) | 0 (0) | > 0.990 |
| Headache | 0.652 | ||
| None | 216 (98.2) | 213 (96.8) | |
| Mild | 3 (1.4) | 5 (2.3) | |
| Moderate | 1 (0.5) | 2 (0.9) | |
| Severe | 0 (0) | 0 (0) | |
| Malaise | 0.573 | ||
| None | 214 (97.3) | 213 (96.8) | |
| Mild | 5 (2.3) | 4 (1.8) | |
| Moderate | 1 (0.5) | 3 (1.4) | |
| Severe | 0 (0) | 0 (0) | |
| Fatigue | 0.766 | ||
| None | 216 (98.2) | 216 (98.2) | |
| Mild | 3 (1.4) | 2 (0.9) | |
| Moderate | 1 (0.5) | 2 (0.9) | |
| Severe | 0 (0) | 0 (0) | |
| Chill | 0.904 | ||
| None | 216 (98.2) | 217 (98.6) | |
| Mild | 3 (1.4) | 2 (0.9) | |
| Moderate | 1 (0.5) | 1 (0.5) | |
| Severe | 0 (0) | 0 (0) | |
| Muscle aches | 0.573 | ||
| None | 214 (97.3) | 213 (96.8) | |
| Mild | 5 (2.3) | 4 (1.8) | |
| Moderate | 1 (0.5) | 3 (1.4) | |
| Severe | 0 (0) | 0 (0) | |
| Arthralgia | 0.606 | ||
| None | 217 (98.6) | 216 (98.2) | |
| Mild | 3 (1.4) | 3 (1.4) | |
| Moderate | 0 (0) | 1 (0.5) | |
| Severe | 0 (0) | 0 (0) |
Values are presented as number (%).
PPV23, 23-valent polysaccharide pneumococcal vaccine; IIV3, trivalent inactivated influenza vaccine; PCV13, 13-valent pneumococcal conjugate vaccine.
Discussion
Concomitant administration of influenza and pneumococcal vaccines has been evaluated from various perspectives. Previous studies have mentioned that the influenza vaccine could be administrated simultaneously with pneumococcal vaccine without affecting immunogenicity and safety. A prospective study of 152 adult participants with chronic respiratory disease suggested that concomitant immunization with PPV23 and influenza vaccine was well tolerated and immunogenically effective at one month after vaccination [17]. Other studies also demonstrated that concomitant PCV13 administration with the influenza vaccine yielded acceptable immunogenicity and safety in the elderly [13,18]. However, there is no study that directly compared the immunogenicity and safety of influenza vaccine given concomitantly with either PCV13 or PPV23 in the elderly.
This study demonstrated that both IIV3+PCV13 and IIV3+PPV23 showed excellent immunogenicity. It is noted that the post-vaccination GMTs of IIV3+PCV13 for A/H1N1 were significantly higher compared with IIV3+PPV23. Although not statistically significant, IIV3+PCV13 showed a relatively higher seroprotection rate, seroconversion rate, and GMT fold for A/H1N1. The higher immune response observed by IIV+PCV13 is not well understood. Such addictive effects could be caused by multifactorial immune interaction. Possible explanation for such phenomenon would be that more immunogenic stimuli of PCV leads to promote the MHC class I and II antigen processing and presentation pathways, which also produce more immunogenic response to influenza vaccine. However, such effect is initiated by local environment and two vaccines were separately administered in each arm, so the assumption could be easily broken. In addition, those could be incidental findings due to the small number of participants in the study group. Further large-scale studies are needed to confirm this finding.
Local and systemic reactions did not differ significantly between IIV3+PCV13 and IIV3+PPV23. Overall, there were no vaccine-related serious adverse events and solicited reactions were mild. Importantly, our study indicates that concomitant vaccination for influenza and pneumococcal disease was safe and generally well tolerated in elderly participants.
This study has several limitations. First, influenza vaccination history and infection status in previous years were not available. Pre-vaccination GMTs were already high in some participants, and could influence immunogenicity. Second, in the present study, a solitary IIV3 administration group was not included. Simultaneous vaccination could influence the immunogenicity of each vaccine. In such situations, having a solitary IIV3 group might be needed to explain the differences of immunogenicity. Third, immunogenicity of pneumococcal vaccine was not assessed. One study which compared PCV13+IIV3 with IIV showed slightly lower immune reaction of PCV13 in concomitant vaccination group [13]. However, other study found no differences [19]. Inconclusive result needs to be confirmed by addition studies.
Current study found similar immunogenicity and safety profiles in response to IIV3 when PCV13 or PPV23 were administered together.
Footnotes
No potential conflict of interest relevant to this article was reported.
This study was supported by a grant from the Korea Healthcare technology R&D Project Ministry of Health & Welfare Republic of Korea (Grant No. A103001).
References
- 1.Walter ND, Taylor TH, Shay DK, et al. Influenza circulation and the burden of invasive pneumococcal pneumonia during a non-pandemic period in the United States. Clin Infect Dis. 2010;50:175–183. doi: 10.1086/649208. [DOI] [PubMed] [Google Scholar]
- 2.Grabowska K, Hogberg L, Penttinen P, Svensson A, Ekdahl K. Occurrence of invasive pneumococcal disease and number of excess cases due to influenza. BMC Infect Dis. 2006;6:58. doi: 10.1186/1471-2334-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mangtani P, Cumberland P, Hodgson CR, Roberts JA, Cutts FT, Hall AJ. A cohort study of the effectiveness of influenza vaccine in older people, performed using the United Kingdom general practice research database. J Infect Dis. 2004;190:1–10. doi: 10.1086/421274. [DOI] [PubMed] [Google Scholar]
- 4.Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med. 2007;357:1373–1381. doi: 10.1056/NEJMoa070844. [DOI] [PubMed] [Google Scholar]
- 5.Mooney JD, Weir A, McMenamin J, et al. The impact and effectiveness of pneumococcal vaccination in Scotland for those aged 65 and over during winter 2003/2004. BMC Infect Dis. 2008;8:53. doi: 10.1186/1471-2334-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vila-Corcoles A, Ochoa-Gondar O, Guzman JA, et al. Effectiveness of the 23-valent polysaccharide pneumococcal vaccine against invasive pneumococcal disease in people 60 years or older. BMC Infect Dis. 2010;10:73. doi: 10.1186/1471-2334-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nichol KL. The additive benefits of influenza and pneumococcal vaccinations during influenza seasons among elderly persons with chronic lung disease. Vaccine. 1999;17(Suppl 1):S91–S93. doi: 10.1016/s0264-410x(99)00114-0. [DOI] [PubMed] [Google Scholar]
- 8.Furumoto A, Ohkusa Y, Chen M, et al. Additive effect of pneumococcal vaccine and influenza vaccine on acute exacerbation in patients with chronic lung disease. Vaccine. 2008;26:4284–4289. doi: 10.1016/j.vaccine.2008.05.037. [DOI] [PubMed] [Google Scholar]
- 9.Christenson B, Hedlund J, Lundbergh P, Ortqvist A. Additive preventive effect of influenza and pneumococcal vaccines in elderly persons. Eur Respir J. 2004;23:363–368. doi: 10.1183/09031936.04.00063504. [DOI] [PubMed] [Google Scholar]
- 10.Fedson DS, Nicolas-Spony L, Klemets P, et al. Pneumococcal polysaccharide vaccination for adults: new perspectives for Europe. Expert Rev Vaccines. 2011;10:1143–1167. doi: 10.1586/erv.11.99. [DOI] [PubMed] [Google Scholar]
- 11.Song JY, Cheong HJ, Heo JY, et al. Outpatient-based pneumococcal vaccine campaign and survey of perceptions about pneumococcal vaccination in patients and doctors. Yonsei Med J. 2013;54:469–475. doi: 10.3349/ymj.2013.54.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honkanen PO, Keistinen T, Kivela SL. Reactions following administration of influenza vaccine alone or with pneumococcal vaccine to the elderly. Arch Intern Med. 1996;156:205–208. [PubMed] [Google Scholar]
- 13.Schwarz TF, Flamaing J, Rumke HC, et al. A randomized, double-blind trial to evaluate immunogenicity and safety of 13-valent pneumococcal conjugate vaccine given concomitantly with trivalent influenza vaccine in adults aged ≥ 65 years. Vaccine. 2011;29:5195–5202. doi: 10.1016/j.vaccine.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 14.Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res. 2004;103:133–138. doi: 10.1016/j.virusres.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 15.Song JY, Cheong HJ, Woo HJ, et al. Immunogenicity and safety of trivalent inactivated influenza vaccine: a randomized, double-blind, multi-center, phase 3 clinical trial in a vaccine-limited country. J Korean Med Sci. 2011;26:191–195. doi: 10.3346/jkms.2011.26.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research. Guidance for industry toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. Rockville: U.S: Department of Health and Human Services; 2007. [Google Scholar]
- 17.Fletcher TJ, Tunnicliffe WS, Hammond K, Roberts K, Ayres JG. Simultaneous immunisation with influenza vaccine and pneumococcal polysaccharide vaccine in patients with chronic respiratory disease. BMJ. 1997;314:1663–1665. doi: 10.1136/bmj.314.7095.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frenck RW, Jr, Gurtman A, Rubino J, et al. Randomized, controlled trial of a 13-valent pneumococcal conjugate vaccine administered concomitantly with an influenza vaccine in healthy adults. Clin Vaccine Immunol. 2012;19:1296–1303. doi: 10.1128/CVI.00176-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song JY, Cheong HJ, Tsai TF, et al. Immunogenicity and safety of concomitant MF59-adjuvanted influenza vaccine and 23-valent pneumococcal polysaccharide vaccine administration in older adults. Vaccine. 2015;33:4647–4652. doi: 10.1016/j.vaccine.2015.05.003. [DOI] [PubMed] [Google Scholar]