Abstract
AIM
To determine if the lymphocyte-to-monocyte ratio (LMR) could be helpful in predicting survival in patients with pancreatic adenocarcinoma.
METHODS
We retrospectively reviewed the medical records of all patients diagnosed with pancreatic adenocarcinoma in the VA North Texas Healthcare System from January 2005 to December 2010. The LMR was calculated from peripheral blood cell counts obtained at the time of diagnosis of pancreatic cancer by dividing the absolute lymphocyte count by the absolute monocyte count. A Univariable Cox regression analysis was performed using these data, and hazard ratios (HR) and 95%CI were calculated. The median LMR (2.05) was used to dichotomize patients into high-LMR and low-LMR groups and the log rank test was used to compare survival between the two groups.
RESULTS
We identified 97 patients with pancreatic adenocarcinoma (all men, 66% white, 30% African-American). The mean age and weight at diagnosis were 66.0 ± 0.9 (SEM) years and 80.4 ± 1.7 kg respectively. Mean absolute lymphocyte and monocyte values were 1.50 ± 0.07 K/μL and 0.74 ± 0.03 K/μL respectively. Mean, median and range of LMR was 2.36, 2.05 and 0.4-12 respectively. In the univariable Cox regression analysis, we found that an increased LMR was a significant indicator of improved overall survival in patients with pancreatic adenocarcinoma (HR = 0.83; 95%CI: 0.70-0.98; P = 0.027). Kaplan-Meier analysis revealed an overall median survival of 128 d (95%CI: 80-162 d). The median survival of patients in the high-LMR (> 2.05) group was significantly greater than the low-LMR group (≤ 2.05) (194 d vs 93 d; P = 0.03), validating a significant survival advantage in patients with a high LMR.
CONCLUSION
The LMR at diagnosis is a significant predictor for survival and can provide useful prognostic information in the management of patients with pancreatic adenocarcinoma.
Keywords: Prognosis, Lymphocyte-to-monocyte ratio, Pancreatic adenocarcinoma, Mortality, Biomarker
Core tip: Pancreatic adenocarcinoma is an aggressive malignancy and many patients are presented with aggressive treatment options at diagnosis; often times they are unsure whether they should take a palliative route or a more aggressive approach to their care. Through a retrospective analysis of patients with pancreatic adenocarcinoma, we found that a higher lymphocyte-to-monocyte ratio is associated with improved survival. The lymphocyte-to-monocyte ratio was collected at diagnosis, and is readily available on routine blood work, making it a simple way to help predict and guide treatment in patients diagnosed with pancreatic adenocarcinoma.
INTRODUCTION
The fourth leading cause of death related to cancer is attributed to pancreatic adenocarcinoma in the United States[1], with an overall 5-year survival of 7.2% and 2.4% in patients with metastatic disease[2]. The disease is generally silent in the early stages and is usually detected once patient develops symptoms from local or distant metastasis. Overall, half of the patients are found to have metastatic disease at diagnosis[1]. Risk factors for pancreatic adenocarcinoma include male sex, elderly age, family history, African American race, obesity, diabetes, tobacco use, and chronic pancreatitis[3,4]. The treatment of pancreatic cancer is dependent on stage of disease, and is divided into three categories: Resectable, locally advanced, and metastatic disease. Locally advanced disease can be treated with neo-adjuvant chemotherapy followed by surgical resection. The mainstay of treatment for metastatic disease is palliative chemotherapy[5,6]. There have been numerous advances in oncologic therapeutics, however improvement of survival in patients with pancreatic cancer has been particularly slow[1].
Currently, there is no effective way to predict treatment response and survival at diagnosis aside from stage of disease. For patients with resected cancer, predictors of survival include resection margins, tumor size, and response to chemo-radiation[7,8]. Given the high morbidity and mortality associated with pancreatic cancer, any prognostic information available to risk stratify patients could be beneficial in planning treatment approaches and palliative discussions.
Inflammation and the body’s cellular immune response have been shown to play an important role in the pathogenesis of malignancy and its progression from primary to metastatic disease[9,10]. These concepts have led the absolute peripheral blood lymphocyte-to-monocyte ratio (LMR) to act as a surrogate biomarker of prognosis in different malignancies, with several studies showing an association between the LMR and survival in multiple myeloma, diffuse large B cell lymphoma, osteosarcoma, non-small cell lung cancer, and breast cancer[11-15].
The primary aim of this study was to determine if the peripheral blood LMR at the time of diagnosis could be used as a prognostic biomarker in patients with pancreatic adenocarcinoma regardless of treatment modality.
MATERIALS AND METHODS
We identified a cohort of patients diagnosed with pancreatic cancer from the Dallas VA tumor registry at the Veteran’s Affairs North Texas Health Care system (VANTHCS) between January 2005 and December 2010. All patients were treated based on the stage of the disease and accepted standard of care treatment protocols which included surgery, chemotherapy, radiation, and palliative stent placement.
We included patients that were only diagnosed with pancreatic adenocarcinoma; other pancreatic tumors such as lymphoma or metastases of other primaries were excluded from the analysis. The study protocol was approved by the VANTHCS Institutional Review Board.
Data collection
Data collected included variables such as age, sex, race, weight, tobacco use, alcohol use, and medical co-morbidities. Specific variables for pancreatic cancer included age at diagnosis, largest diameter of tumor size seen on cross-sectional imaging and survival time in days (using a cutoff date of 10/11/2014 when the date of death was not available). Lab values including CA 19-9, CEA, white blood cell count, platelets, absolute lymphocyte count, lymphocyte percentage, absolute monocyte count, and monocyte percentage were all collected at or within one week of diagnosis.
LMR
The LMR was calculated by dividing the absolute lymphocyte count by the absolute monocyte count on the same blood draw that was obtained at the initial diagnosis of pancreatic cancer and prior to the initiation of any treatment.
Statistical analysis
Univariable Cox regression statistical analysis was performed to determine if LMR was a predictor of survival in patients with pancreatic adenocarcinoma; hazard ratios (HR) and 95%CI were calculated. A P < 0.05 was considered statistically significant. The median LMR was used to dichotomize patients into two groups: Patients with high-LMR and low-LMR. A Kaplan-Meier analysis with log rank test was used to compare survival between the two groups. The association between variables in the subgroups was evaluated by the χ2 test for categorical variables, the t test for continuous variables, or the Fisher’s Exact test.
These analyses were performed using SAS (version 9.2 software, The SAS Institute, Cary, NC) and R (version 2.15.1, the R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
The overall baseline demographics, histopathologic characteristics, and stage are outlined in Table 1. There were 109 total patients in the Dallas VA Tumor registry that had any type of pancreatic cancer diagnosed between January 2005 and December 2010. Twelve patients with pancreatic neuroendocrine tumors were excluded. In the final analysis, a total of ninety seven patients with pancreatic adenocarcinoma were included (demographics were 66% white, 30% African-American; all were male subjects).
Table 1.
Baseline demographics pancreatic adenocarcinoma (n = 97) n (%)
| Characteristic | Mean ± SEM |
| Age | 66 ± 0.9 |
| Weight at dx (lbs) | 80. 4 ± 1.7 |
| LMR | 2.36 ± 0.16 |
| CA 19-9 | 17030.2 ± 8861 |
| CEA | 960 ± 657 |
| Tumor size (cm) | 4.13 ± 0.2 |
| WBC | 9.1 ± 0.5 |
| Platelets | 276.8 ± 12.2 |
| ALC K/μL | 1.5 ± 0.07 |
| AMC K/μL | 0.74 ± 0.03 |
| Race | |
| White | 64 (66) |
| Black | 29 (30) |
| Other | 4 (4) |
| Location | |
| Head | 64 (71) |
| Body | 10 (11) |
| Tail | 16 (18) |
| Stage | |
| I | 1 (1) |
| II | 23 (24) |
| III | 14 (14) |
| IV | 59 (61) |
| Treatment | |
| Surgery | 22 (23) |
| Stent | 38 (39) |
| Any chemo/rad | 44 (45) |
| Palliative chemotherapy | 36 (37) |
| Neoadjuvant chemotherapy | 3 (3) |
| Adjuvant chemotherapy | 10 (10) |
| Risk factors | |
| Alcohol | 53 (55) |
| Tobacco | 76 (78) |
The stage at presentation was I (1%), II (24%), III (14%), and IV (61%). Patients had different presenting symptoms including weight loss (53%), jaundice (44%), poor appetite (21%) and abdominal pain (58%). Treatment included surgery (22%), neo-adjuvant therapy (3%) and palliative chemotherapy (37%) (Table 1). Kaplan-Meier survival analysis revealed an overall median survival for patients with pancreatic adenocarcinoma of 128 d (95%CI: 80-162 d).
Ninety-three of the 97 patients with pancreatic adenocarcinoma (96%) had absolute peripheral blood lymphocyte and monocyte values available at diagnosis to calculate the LMR. Mean absolute lymphocyte and mean absolute monocyte values were 1.50 ± 0.07 K/uL and 0.74 ± 0.03 K/μL respectively. Mean, median and range of LMR was 2.36, 2.05 and 0.4-12 respectively.
Univariable Cox regression analysis showed that an increased LMR was a significant indicator of improved overall survival in patients with pancreatic adenocarcinoma (HR = 0.83; 95%CI: 0.70-0.98; P = 0.027). Moreover, a high LMR in this group was significantly associated with a lower risk of early mortality, i.e., survival < 6 mo (OR = 0.66; 95%CI: 0.46-0.95; P = 0.025). The median survival of patients in the high-LMR group (> 2.05) was significantly greater than the low-LMR group (≤ 2.05) (194 d vs 93 d; P = 0.03) (Figure 1).
Figure 1.
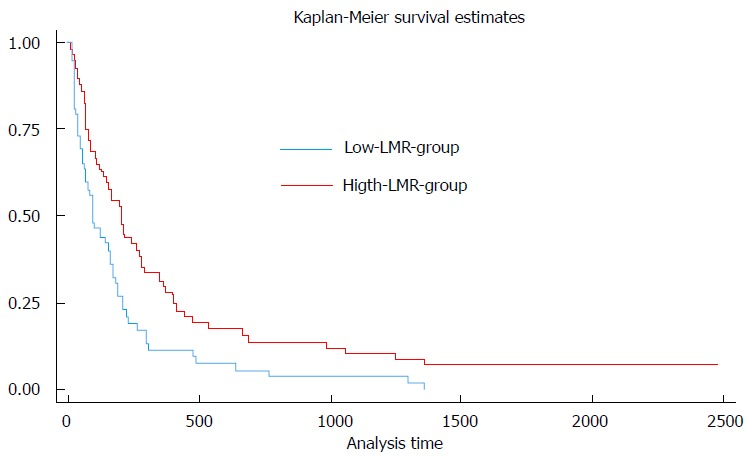
Kaplan meier survival curves of patients with low and high lymphocyte-to-monocyte ratio.
To investigate the value of LMR in metastatic disease (stage IV), a uni-variable logistic regression analysis was performed in this group. There was no significant association between LMR and development of metastatic disease (OR = 0.91; P = 0.476). The area under the ROC curve was 0.609 (Figure 2), suggesting that LMR may be a poor marker for the prediction of metastatic disease.
Figure 2.
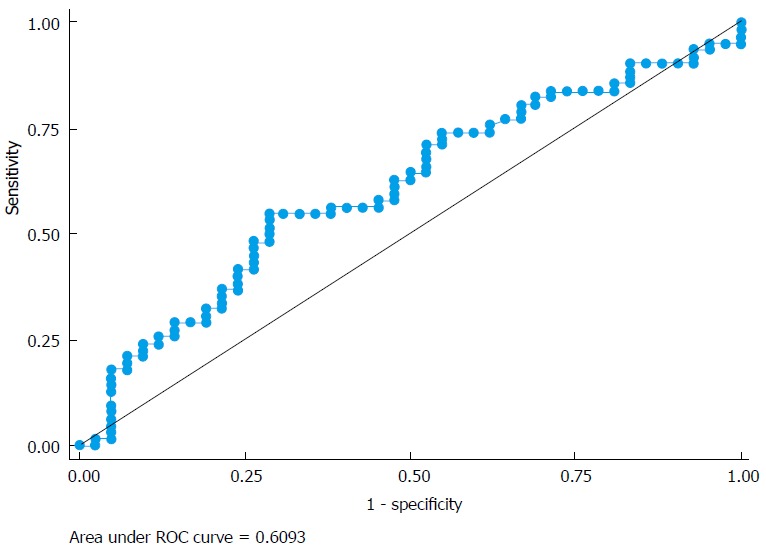
Price rate of change curve of the accuracy of lymphocyte-to-monocyte ratio in prediction of metastatic disease.
A uni-variable analysis of demographic and clinical variables between the high-LMR and low-LMR was performed to further characterize factors that could affect survival between the two groups. There was a marginally significant difference in the percentage receiving surgery in the high-LMR groups vs low-LMR group (P = 0.05) as well as in race between both groups (P = 0.05). There was no statistical significant difference between patients receiving chemo-radiation (P = 0.4) or stenting (P = 1). Furthermore, there was no difference in demographic variables such as age (P = 0.5), weight (P = 0.4), or risk factors such as tobacco (P = 0.8) or alcohol (P = 1.0) usage between the two groups. Analysis of clinical variables such as stage at presentation, location of tumor, mean CEA levels, and CA 19-9 levels between both groups did not reveal any significant difference (Table 2).
Table 2.
Clinical variables in patients with high and low lymphocyte-to-monocyte ratio
| LMR ≤ 2.05 (n = 50) | LMR > 2.05 (n = 43) | P value | |
| Chemoradiation | 20 | 21 | 0.4 |
| Surgery | 7 | 14 | 0.05 |
| Stent | 19 | 16 | 1.0 |
| Stage | 0.2 | ||
| Stage 1 | 0 | 1 | |
| Stage 2 | 8 | 14 | |
| Stage 3 | 8 | 5 | |
| Stage 4 | 34 | 23 | |
| Location | 0.4 | ||
| Head | 35 | 27 | |
| Body | 3 | 6 | |
| Tail | 9 | 6 | |
| Race | 0.05 | ||
| White | 36 | 25 | |
| Black | 11 | 18 | |
| Other | 3 | 0 | |
| CEA (± SEM) | 1075 ± 1041 | 884 ± 868 | 0.9 |
| CA 19-9 (± SEM) | 24957 ± 17470 | 10162 ± 3448 | 0.4 |
| Age (± SEM) | 66.6 ± 1.2 | 65.3 ± 1.4 | 0.5 |
| Weight (± SEM) | 79.3 ± 2.2 | 82.2 ± 2.5 | 0.4 |
| Alcohol | 27 | 24 | 1.0 |
| Tobacco | 38 | 34 | 0.8 |
LMR: Lymphocyte-to-monocyte ratio.
DISCUSSION
In this study we show that a higher LMR obtained from a peripheral blood count at the time of diagnosis is a predictor of improved survival in patients with pancreatic adenocarcinoma.
The LMR represents the balance between anti-tumorigenic lymphocytes and pro-tumorigenic monocytes, and may reflect the body’s immune response to cancer and host-specific cancer aggressiveness. The T-lymphocytes of the native immune system play a vital role in suppressing anti-tumor immune responses and inducing apoptosis in tumor cells; low levels of T-lymphocytes have been implicated in a poor immune response to cancers[9,16,17]. Monocytes have been implicated in tumorigenesis, including differentiation into tumor-associated macrophages that support tumor invasion, angiogenesis and suppression of the body’s own immune response against the tumor cells[10,18,19]. Various studies have shown that the lymphocytes have an anti-inflammatory function and their role in impeding progression of tumor may be vital in the immune surveillance of different types of malignancies. Lymphocytes have different roles in identifying and eliminating tumor cells. This phenomenon is sometimes referred to as “immunoediting”, and includes a complex interplay of various cells such as the NK cells, the NKT cells, macrophages, CD4 T cells and CD8 T cells. Several studies have shown that high numbers of CD8 T cells within the tumor portend a better prognosis[20]. Further testing of these cells in a study including patients with pancreatic adenocarcinoma revealed FOXP3+ protein on immunohistochemical staining[21]. This was further evaluated in a study, which looked at the lymphocyte density and the correlation with lymph node metastasis. They found out that the presence of FOXP3+ lymphocyte was higher in patients who had a higher histological grade of tumor, lymph node metastasis, and advanced stage tumors (stage III and IV vs stage I and II)[22]. While the studies were not prospective in nature, they do validate the importance of these inflammatory cells in dictating the prognosis of these cancers.
Moreover, cytokines released by lymphocytes have roles in both promoting and suppressing a cancer. Haabeth et al[23] conducted a study measuring the cytokine response in mice against cancers (myeloma and B-cell lymphoma). They found that inflammation driven by tumor specific Th1, allowed release of IFN-gamma which stimulated macrophages that were cytotoxic to the cancer cells. The CD4+ Th1 cells also help cytotoxic T cells in tumor rejection. On the other hand, the CD4+ Th2 cells are implicated in production of cytokines leading to B-cell activation. Similarly, Ling et al. showed that high numbers of Th1 lymphocytes in tumor tissue was associated with improved prognosis in patients with colorectal cancer[24].
The prognostic ability of the LMR has been demonstrated in various malignancies[11-15]. However, the exact utility of the LMR for primary pancreatic adenocarcinoma is unclear given the limited data available to date. Li et al[25] evaluated the prognostic utility of the LMR in patients with pancreatic adenocarcinoma in the People’s Republic of China but only included patients who underwent pancreatic resection and excluded patients who received adjuvant treatment, had significant co-morbid conditions or a life expectancy of < 6 mo. In addition, the preoperative LMR was used and not the LMR at diagnosis. They found that an elevated preoperative LMR was associated with longer survival. Fujiwara et al[26] evaluated the postoperative LMR exclusively in patients who received pancreatic resections in Japan. They also reported an association between higher LMR and disease free survival.
In our study we included all adult patients diagnosed with pancreatic adenocarcinoma regardless of co-morbidities, life expectancy, functional status or intervention modality. Our study is the first study evaluating the utility of the LMR in an American cohort and shows that a higher LMR obtained at diagnosis of pancreatic cancer, regardless of the patient’s functional status, various clinical factors or patient demographics validates a significant survival advantage. This information may be used in conjunction with other clinical factors to help in discussing prognosis and/or palliative options with patients as well as aid in scenarios where pateints and providers decide on surgery vs neoadjuvant chemotherapy for borderline resectable tumors.
There are several limitations of this study. These include problems inherent to a retrospective study design such as treatment bias, a limited number of patients, as well as a unique patient population comprising exclusively of veteran male patients. The strengths of the study include a comprehensive multidisciplinary evaluation of all patients in a tertiary care center with follow-up data available for each patient included in the analysis.
There is a role of immune-targeted therapies in the future, as it is clear that specific inflammatory cells have an impact on immune surveillance of tumors. Immunotherapies including chemicals that resemble cytokines can be used to up-regulate the cancer fighting cells. Clarifying the specific type of immune cells and chemical cytokines that attract tumor suppressing cells requires further research and understanding of the tumor biology, specifically for the different types of malignancies. Pancreatic adenocarcinoma, despite being one of the more aggressive cancers, still is in the nascent stages of research and investigators are continuing to learn the tumor biology and immunologic effects.
In the future, a large multicenter prospective trial would be beneficial to confirm our findings and validate it for routine use in the prognostication of patients with pancreatic cancer. The cutoff level of the LMR has varied in different studies, with each study using a level specific to their cohort. In the future a single cutoff value for the LMR would need to be validated for further research purposes and clinical use. Moreover, the significance of LMR within various disease stages or a specific treatment modality (such as surgery, chemotherapy and radiation) could be further explored in adequately powered research studies.
In conclusion, the LMR is an easily acquired, minimally invasive, and inexpensive biomarker that may reflect the body’s immune response to cancer and host-specific cancer aggressiveness. Our study shows that a high LMR predicts better overall survival for patients with pancreatic adenocarcinoma and can be used by clinicians and patients as a marker for prognosis.
COMMENTS
Background
Pancreatic adenocarcinoma is an aggressive malignancy and many patients are presented with aggressive treatment options at diagnosis. Inflammation plays an important role in cancer progression and metastasis, and the authors hypothesized that the lymphocyte to monocyte ratio may be a potential surrogate marker of prognosis, helping the patients make difficult decisions regarding which treatment option to pursue. Lymphocytes can be cytotoxic to tumor cells and can induce apoptosis in them, whereas monocytes have properties that promote tumorigenesis. These features might explain why a high lymphocyte-to-monocyte ratio (LMR) in peripheral blood has been found to be a favorable prognostic marker for a number of malignancies.
Research frontiers
Several malignancies have shown to have a favorable prognosis with a high peripheral blood LMR. Further research in determining the actual cellular mechanisms regarding the cytotoxic and apoptotic effects of inflammatory cells in different malignancies is where the basic science aspect would be beneficial. Also, validating a certain cutoff point for the LMR will allow clinicians to use the LMR in practice as a prognostic marker.
Innovations and breakthroughs
The LMR has been shown to be of prognostic significance in different malignanices such as breast cancer, multiple myeloma, lymphomas, osteosarcomas, and lung cancers. There are also other inflammatory markers in investigation such as the neutrophil to lymphocyte ratio, which has also been shown to have some prognostic significance.
Applications
They can use the LMR as a surrogate marker of prognosis in patients with pancreatic adenocarcinoma. It is available on routine blood work as they can calculate the LMR from a peripheral blood draw. The value of the LMR, if high, suggests a better prognosis, and may help guide treatment decisions.
Terminology
LMR is the absolute lymphocyte count divided by the absolute monocyte count.
Peer-review
The paper is well-written.
Footnotes
Institutional review board statement: The study was reviewed and approved by the VA North Texas Health Care System Institutional Review Board.
Informed consent statement: All study data was de-identified and was approved by the institutional review board.
Conflict-of-interest statement: The authors declare no conflicts of interest regarding this manuscript.
Data sharing statement: No data were created no data are available.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: September 1, 2016
First decision: September 29, 2016
Article in press: December 14, 2016
P- Reviewer: Abdou AG, Aktas S, Luchini C S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol. 2009;6:699–708. doi: 10.1038/nrgastro.2009.177. [DOI] [PubMed] [Google Scholar]
- 3.Eskander MF, Bliss LA, Tseng JF. Pancreatic adenocarcinoma. Curr Probl Surg. 2016;53:107–154. doi: 10.1067/j.cpsurg.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Midha S, Chawla S, Garg PK. Modifiable and non-modifiable risk factors for pancreatic cancer: A review. Cancer Lett. 2016;381:269–277. doi: 10.1016/j.canlet.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 5.Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85. doi: 10.1016/S0140-6736(16)00141-0. [DOI] [PubMed] [Google Scholar]
- 6.Conroy T, Bachet JB, Ayav A, Huguet F, Lambert A, Caramella C, Maréchal R, Van Laethem JL, Ducreux M. Current standards and new innovative approaches for treatment of pancreatic cancer. Eur J Cancer. 2016;57:10–22. doi: 10.1016/j.ejca.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 7.Chang DK, Johns AL, Merrett ND, Gill AJ, Colvin EK, Scarlett CJ, Nguyen NQ, Leong RW, Cosman PH, Kelly MI, et al. Margin clearance and outcome in resected pancreatic cancer. J Clin Oncol. 2009;27:2855–2862. doi: 10.1200/JCO.2008.20.5104. [DOI] [PubMed] [Google Scholar]
- 8.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, Sauter PK, Coleman J, Hruban RH, Lillemoe KD. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 10.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin SJ, Roh J, Kim M, Jung MJ, Koh YW, Park CS, Yoon DH, Suh C, Park CJ, Chi HS, et al. Prognostic significance of absolute lymphocyte count/absolute monocyte count ratio at diagnosis in patients with multiple myeloma. Korean J Pathol. 2013;47:526–533. doi: 10.4132/KoreanJPathol.2013.47.6.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe R, Tomita N, Itabashi M, Ishibashi D, Yamamoto E, Koyama S, Miyashita K, Takahashi H, Nakajima Y, Hattori Y, et al. Peripheral blood absolute lymphocyte/monocyte ratio as a useful prognostic factor in diffuse large B-cell lymphoma in the rituximab era. Eur J Haematol. 2014;92:204–210. doi: 10.1111/ejh.12221. [DOI] [PubMed] [Google Scholar]
- 13.Liu T, Fang XC, Ding Z, Sun ZG, Sun LM, Wang YL. Pre-operative lymphocyte-to-monocyte ratio as a predictor of overall survival in patients suffering from osteosarcoma. FEBS Open Bio. 2015;5:682–687. doi: 10.1016/j.fob.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin GN, Peng JW, Xiao JJ, Liu DY, Xia ZJ. Prognostic impact of circulating monocytes and lymphocyte-to-monocyte ratio on previously untreated metastatic non-small cell lung cancer patients receiving platinum-based doublet. Med Oncol. 2014;31:70. doi: 10.1007/s12032-014-0070-0. [DOI] [PubMed] [Google Scholar]
- 15.Ni XJ, Zhang XL, Ou-Yang QW, Qian GW, Wang L, Chen S, Jiang YZ, Zuo WJ, Wu J, Hu X, et al. An elevated peripheral blood lymphocyte-to-monocyte ratio predicts favorable response and prognosis in locally advanced breast cancer following neoadjuvant chemotherapy. PLoS One. 2014;9:e111886. doi: 10.1371/journal.pone.0111886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann TK, Dworacki G, Tsukihiro T, Meidenbauer N, Gooding W, Johnson JT, Whiteside TL. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin Cancer Res. 2002;8:2553–2562. [PubMed] [Google Scholar]
- 18.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 20.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shibuya KC, Goel VK, Xiong W, Sham JG, Pollack SM, Leahy AM, Whiting SH, Yeh MM, Yee C, Riddell SR, et al. Pancreatic ductal adenocarcinoma contains an effector and regulatory immune cell infiltrate that is altered by multimodal neoadjuvant treatment. PLoS One. 2014;9:e96565. doi: 10.1371/journal.pone.0096565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang Y, Du Z, Yang F, Di Y, Li J, Zhou Z, Pillarisetty VG, Fu D. FOXP3+ lymphocyte density in pancreatic cancer correlates with lymph node metastasis. PLoS One. 2014;9:e106741. doi: 10.1371/journal.pone.0106741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haabeth OA, Lorvik KB, Hammarström C, Donaldson IM, Haraldsen G, Bogen B, Corthay A. Inflammation driven by tumour-specific Th1 cells protects against B-cell cancer. Nat Commun. 2011;2:240. doi: 10.1038/ncomms1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ling A, Lundberg IV, Eklöf V, Wikberg ML, Öberg Å, Edin S, Palmqvist R. The infiltration, and prognostic importance, of Th1 lymphocytes vary in molecular subgroups of colorectal cancer. J Pathol Clin Res. 2016;2:21–31. doi: 10.1002/cjp2.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li GJ, Xu HW, Ji JJ, Yang F, Gao BQ. Prognostic value of preoperative lymphocyte-to-monocyte ratio in pancreatic adenocarcinoma. Onco Targets Ther. 2016;9:1085–1092. doi: 10.2147/OTT.S96707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujiwara Y, Misawa T, Shiba H, Shirai Y, Iwase R, Haruki K, Furukawa K, Futagawa Y, Yanaga K. Postoperative peripheral absolute blood lymphocyte-to-monocyte ratio predicts therapeutic outcome after pancreatic resection in patients with pancreatic adenocarcinoma. Anticancer Res. 2014;34:5163–5168. [PubMed] [Google Scholar]


