Abstract
Background
Previous studies have shown that xanthine oxidase inhibitors (XOI) might improve outcome for patients with cardiovascular disease. However, more evidence is required.
Methods and results
We published a meta‐analysis of trials conducted before 2014 examining the effects of XOI on mortality in patients with cardiovascular disease. At least two further trials (N = 323 patients) have since been published. Accordingly, we repeated our analysis after a further search for randomized controlled trials of XOI in PubMed/MEDLINE, EMBASE, and Cochrane Databases. We identified eight relevant trials with 1031 patients. The average age of the patients was 61 years and 68% were men (one study did not report gender). There were 57 deaths in these eight trials, 26 in those assigned to XOI, and 31 in those assigned to the control. The updated meta‐analysis could not confirm a reduction in mortality for patients assigned to XOI compared with placebo (odds ratio 0.84) but 95% confidence intervals were wide (0.48–1.47).
Conclusions
This updated meta‐analysis does not suggest that XOI exert a large reduction in mortality but also cannot exclude the possibility of substantial harm or benefit.
Keywords: Xanthine oxidase inhibition, Cardiovascular disease, Mortality, Systematic review, Meta‐analysis
Introduction
Xanthine oxidase inhibitors (XOIs) reduce the production of uric acid (UA), its serum concentration, and UA crystal deposition in joints, thereby reducing the risk of recurrent gout. The production of UA by xanthine oxidase also generates free radicals that might adversely affect mitochondrial function and ATP production. XOIs might reduce free radical production, leading to improved left ventricular (LV) function and reverse LV remodeling and renal function.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 There is a substantial literature suggesting that serum UA concentrations are associated with worse cardiovascular outcomes.13, 14, 15 However, UA is excreted by the kidney and therefore a marker of renal function which is also strongly associated with cardiovascular outcome. UA is also an anti‐oxidant.16 Accordingly, there are theoretical reasons why XOI could improve, worsen, or have no effect on cardiovascular outcomes.17
We previously reported a systematic review of the effects of XOI on mortality in randomized controlled trials (RCTs) conducted in patients with cardiovascular disease.18 The overall odds ratio (OR) and 95% confidence intervals (CI) were: 0.52 (0.19–1.40) suggesting that there might be a large benefit for XOIs, but with insufficient evidence to be certain. The recent EXACT‐HF Study adds a substantial new dataset using XOI for patients with heart failure (HF),19 and we were also aware of a further recent study.20 We therefore updated our systematic review.
Methods
The study was designed according to the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) Statement.21 Inclusion criteria were as follows: RCTs combined with XOI use. Participants were patients with known cardiovascular disease. Observational studies and studies that did not report mortality were excluded. There were no language restrictions.
The PubMed/Medline, Embase, and Cochrane Central Register of Controlled Trials database were searched until September 2015. Studies were identified with the following headings: allopurinol, oxypurinol, xanthine oxidase, cardiovascular disease, clinical trial, and randomized controlled trial. We also searched reference lists of the retrieved articles to identify other eligible studies.
Two investigators independently reviewed all titles, or titles and abstracts from the search results to identify articles that met the inclusion criteria. Selected trials were compared, and disagreement was resolved by review team discussion and consensus. If any of the eligibility criteria were not met, the article was excluded. If results were incomplete or unclear, attempts were made to contact the study authors. Articles finally selected for the review were checked to avoid inclusion of data published in duplicate. Relevant information was collected on baseline characteristics, New York Heart Association (NYHA) functional classification, background cardiovascular disease, study interventions, clinical outcomes at baseline, and end follow‐up. One study22 combined death and nonfatal myocardial infarction/stroke as a major adverse cardiac event and did not report them separately. We included these events in the meta‐analysis. Of 183 articles identified by the initial search, 42 were retrieved for more detailed evaluation. Only eight studies were included in the review largely because of absence of mortality data.
Statistical analysis was carried out using the Review Manager software (RevMan version 5.5). OR with 95% CI were used to assess all‐cause mortality between the two treatment groups. Heterogeneity was assessed using chi‐squared tests as well as I‐squared test. Forest plots are used to represent the results generated from the random‐effects meta‐analysis graphically. The pooled OR and the degree of heterogeneity are presented. Publication bias was minimized by comprehensive literature searching. In addition, a graphical display (funnel plot) of the size of the treatment effect against the precision of the trial (one/standard error) was used to investigate publication bias. However, the funnel plot approach was not used where the number of included studies was small.
Results
Eight studies were identified including 1031 patients (Table 1). The average age of the patients was 61 ± 8 years, and 68% were men excluding 113 patients in one study that did not report gender. Where NYHA class was reported, most patients were in class III or IV. Common adverse effects in the trials are shown in Table 2.
Table 1.
Patient characteristics
| Study | Treatment (N) | Control (N) | Age (years) | Men (%) | Follow‐up | NYHA class III/IV (%) | LVEF | AF or flutter (%) | Systolic PB | HR (%) | CV disease |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Coghlan 199423 | Allopurinol (300 mg) N = 25 | Placebo N = 25 | 58 ± 2 | 84% | 24 h | 80% | 4% of patients <40% | — | — | — | Angina undergoing first CABG operation |
| Gavin 200526 | Allopurinol (300 mg/day) N = 25 | Placebo N = 25 | 67 ± 9 | 77% | 3 months (controlled crossover trial with one month washout in between) | 27% | — | 28% | — | — | CHF (NYHA class II and III) and LV systolic dysfunction |
| Hare 200827 | Oxypurinol (600 mg/day) N = 203 | Placebo N = 202 | 65 ± 13 | 73% | 24 weeks | 96% | <40% | — | — | — | Symptomatic HF (NYHA class III to IV) and LVEF ≤ 40% |
| Talwar 201024 | Allopurinol (300 mg the night before the surgery) N = 25 | Placebo N = 25 | 31 ± 13 | 54% | 24 h | — | — | 24% | — | — | Valvular heart disease undergoing open‐heart surgery |
| Rentoukas 201022 | Allopurinol (loading dose 400 mg followed by 100 mg for 1 month) N = 21 | Placebo N = 19 | 64 ± 10 | 73% | 1 month | — | 42 ± 7% | — | — | — | Admitted with ST segment elevation myocardial infarction |
| Goicoechea 201042 | Allopurinol (100 mg/day) N = 57 | Usual therapy N = 56 | 72 ± 9 | — | 24 months | — | — | — | 146 ± 18 | — | Presence of renal disease (eGFR < 60 mL/min) |
| Taheraghdam 201420 | Allopurinol (200 mg/day) N = 35 | Placebo N = 35 | 68.9 ± 11.33 | 36% | 3 months | — | — | — | — | — | Acute ischemic stroke diagnosed during admission |
| Givertz 201519 |
Allopurinol (600 mg daily) N = 128 |
Placebo N = 125 | 63 (53–73) | 82% | 24 weeks | 134 (53%) | <40% | 49% | 110 ± 16 | 74 ± 13 | Symptomatic HF, LVEF ≤ 40% and uric acid levels ≥ 9.5 mg/dL |
Table 2.
Adverse effects reported with the use of allopurinol in chronic heart failure
| Study | Treatment | Control |
Treatment adverse effects (% of patients) |
Control adverse effects (% of patients) |
|---|---|---|---|---|
| Coghlan 199423 |
Allopurinol (300 mg) Each patient received one tablet at 8 pm on the evening before the operation and one tablet with their premedication (1 h before the operation). |
Placebo | Two required sodium nitroprusside infusion to maintain systolic blood pressure below 120 mm Hg | One required sodium nitroprusside infusion to maintain systolic blood pressure below 120 mm Hg |
| Gavin 200526 |
Allopurinol (300 mg/day) |
Placebo | Two patients developed a skin rash |
One patient developed alopecia, one developed severe back pain, one developed diabetes |
| Hare 200827 |
Oxypurinol (600 mg/day) |
Placebo | — | — |
| Talwar 201024 |
Allopurinol (300 mg the night before the surgery) |
Placebo |
The highest value of troponin T = 3.5 ng/mL; After 24 h the highest value of troponin T = 4.0 ng/mL |
The highest value of troponin T = 3.2 ng/mL; After 24 h the highest value of troponin T = 4.0 ng/mL |
| Rentoukas 200922 |
Allopurinol (loading dose 400 mg followed by 100 mg for 1 month) |
Placebo | — | — |
| Goicoechea 201042 |
Allopurinol (100 mg/day) |
Usual therapy |
No hematologic alterations or serious adverse events in relation to allopurinol treatment appeared in the follow‐up study |
— |
| Taheraghdam 201420 |
Allopurinol (200 mg/day) |
Placebo |
Skin rashes or dermatitis 4 (11.4%) Nausea 6 (17.1%) Abdominal pain 4 (11.4%) Diarrhea 4 (11.4%) Hepatic enzyme disorders 2 (5.7%) Drowsiness 4 (11.4%) Paresthesia 1 (2.8%) Headache 7 (20%) |
Skin rashes or dermatitis 0 Nausea 5 (14.2%) Abdominal pain 2 (5.7%) Diarrhea 3 (8.5%) Hepatic enzyme disorders 1 (2.8%) Drowsiness 5 (14.2%) Paresthesia 1 (2.8%) Headache 9 (25.7%) |
Compared with placebo, initiation of XOI prior to coronary artery bypass graft (CABG) surgery improved recovery of cardiac function,23 maintained normal sinus rhythm after open‐heart surgery,24 and might have reduced heart muscle injury and clinical outcomes after percutaneous coronary intervention (PCI)25 in a series of small studies. One new study20 showed that patients with high serum urate assigned to allopurinol for three months after an acute ischemic stroke had improved functional outcome evaluated by a modified Rankin scale (OR = 4.65, p = 0.014). However, there was no effect on mortality compared with placebo (p = 0.28).
For patients with chronic HF, Gavin and Struthers26 showed that the plasma B‐type natriuretic peptide (BNP) concentrations fell after 2–3 months treatment with allopurinol compared with placebo. However, Hare et al. 27 reported that there was no effect of XOI on NYHA class over 24 weeks (p = 0.42). The recent EXACT‐HF study19 enrolled 253 patients with left ventricular ejection fraction (LVEF) ≤40% and randomly allocated them to allopurinol or placebo. There was no effect of allopurinol on the primary composite end point of survival, worsening HF, and patient global assessment at 24 weeks or LVEF despite a reduction in serum acid.
The two new studies19, 20 almost double the number of deaths reported in trials of XOI in cardiovascular disease. In the meta‐analysis, there are now 1031 patients with 57 events. The mean duration of follow‐up in the studies was about 5.4 months. Although the OR for mortality is 0.84 in favour of XOI, the 95% CI was wide (0.48–1.47) (Figure 1). A funnel plot for publication bias (Figure 2) suggests that it is unlikely that XOI have a striking effect on mortality but that substantial benefit or harm cannot be ruled out. Further analyses, confined to studies or subgroups with hyperuricaemia, provide a similar result.
Figure 1.
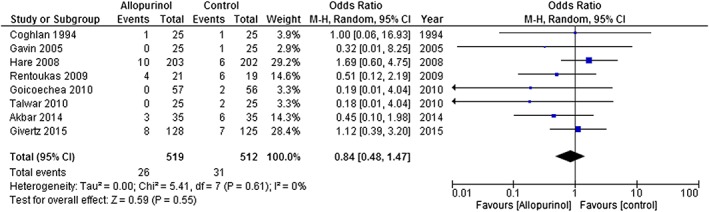
Forest plots of odds ratios for risk of mortality with the exception of the trial by Rentoukas (2009) that only reported a composite of death, stroke, and myocardial infarction. Removing this trial did not materially alter the overall result [odds ratio and 95% confidence interval: 0.92 (0.50–1.68)].
Figure 2.
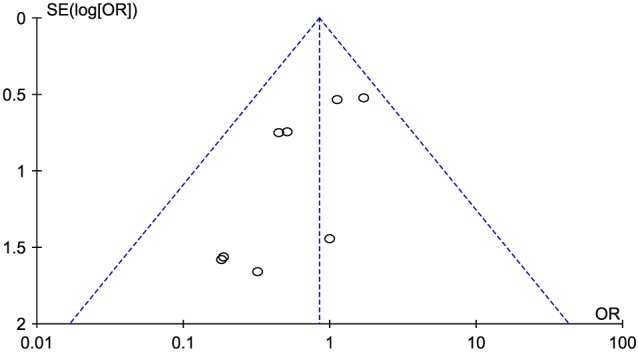
Funnel plot of estimates of the intervention effect from individual studies. Effect estimates from small studies are nearer the bottom of the graph with larger studies nearer the top. There is evidence of publication bias with several small studies suggesting a large benefit but no small studies suggesting substantial harm. Larger studies (although they are still small) in this meta‐analysis are closer to neutrality. This may lead to an overestimate of benefit in this meta‐analysis.43, 44
Discussion
We found that use of XOI was associated with a reduction of serum UA but there was no convincing evidence that this translated into a reduction in mortality in patients with cardiovascular disease.
Many studies have shown that elevated serum concentrations of UA are a risk factor for cardiovascular events28, 29 with the notable exception of Framingham Heart Study that concluded that UA did not have a causal role in the development of coronary heart disease and was not associated with either cardiovascular or all‐cause mortality.30 UA can have both pro‐ and anti‐oxidant effects.31, 32 Under normal conditions, reactive oxygen species are generated by xanthine oxidase that may impair nitric oxide production, and consequently induce endothelial dysfunction, and impair mitochondrial ATP production.33 UA may also stimulate production of C‐reactive protein, inhibit endothelial cell proliferation, which can also cause endothelial dysfunction,34 induce smooth muscle cell proliferation, and increase the production of angiotensin II.35 However, UA is excreted by the kidney. Renal impairment will lead to both an increase in serum UA and is associated with an increase in cardiovascular risk. Whether, serum UA mediates some of the relationship between renal impairment and cardiovascular risk is uncertain; serum UA may just be an alternative to serum creatinine as a measure of renal dysfunction.
Filipatos et al. 36 showed an association between hyperuricaemia and all‐cause mortality (HR with 95%CI: 1.4 (1.1–1.8), p = 0.005) and hospitalization for HF (HR with 95%CI: 1.5 (1.2–1.9), p = 0.001) in patients with HF who did not have chronic kidney disease (CKD), but this association did not persist in those with CKD. They hypothesized that it was over‐production of UA with attendant oxidative stress rather than simply an elevated plasma concentration of UA that accounted for these observations. Ekundayo et al. 37 showed similar associations amongst community dwelling older adults.
Several observational studies suggest that allopurinol use is associated with lower mortality but association does not prove causality.38, 39 Other treatments for gout have also been purported to have effects on cardiovascular outcome.40 A recent study41 suggested that colchicine use was associated with a 73% reduction in all‐cause mortality over 16.5 months in patients with gout. However, colchicine users were more likely to take allopurinol (42% vs. 16%, p < 0.0001). Allopurinol may just be a surrogate marker for a different pattern or quality of care rather than an effective treatment to reduce cardiovascular risk. Differences amongst studies might also reflect the dose of allopurinol used.
There are several limitations to this analysis. Most RCTs were single‐centre which are more prone to investigator biases, include few patients, and have a relatively short follow‐up time and very few events. Robust conclusions should not be drawn from such data. The trials also studied heterogeneous groups of patients who were at different risks of mortality both in terms of rate and cause. The heterogeneity among the patients can explain the inconclusive results and the wide confidence limits of the analysis. The dose of XOI might also be important. Doses of allopurinol ranged from 100 mg⁄day to 600 mg⁄day. There might be a steep dose–response relationship between allopurinol and its cardiovascular effects.
In conclusion, studies of XOI conducted in patients with cardiovascular disease do not suggest a large benefit but also do not exclude either substantial harm or benefit. The optimal dose of XOI is uncertain. Further large, multi‐centre randomized controlled clinical trials are required to ensure the safety of using long‐term XOI in patients with cardiovascular disease and hyperuricaemia and to confirm that this leads to a reduction in potential UA‐related disease, such as gout and renal dysfunction. This will demonstrate whether or not XOI reduce cardiovascular morbidity and mortality. Future studies might stratify patients not only on serum UA concentration but also renal function to tease out the respective roles of high serum UA concentration and high UA production.
Conflict of interest
None declared.
Funding
None.
Zhang, J. , Dierckx, R. , Mohee, K. , Clark, A. L. , and Cleland, J. G. (2017) Xanthine oxidase inhibition for the treatment of cardiovascular disease: an updated systematic review and meta‐analysis. ESC Heart Failure, 4: 40–45. doi: 10.1002/ehf2.12112.
References
- 1. Reyes AJ. The increase in serum uric acid concentration caused by diuretics might be beneficial in heart failure. Eur J Heart Fail 2005; 7: 461–467. [DOI] [PubMed] [Google Scholar]
- 2. Struthers AD, Donnan PT, Lindsay P, McNaughton D, Broomhall J, MacDonald TM. Effect of allopurinol on mortality and hospitalisations in chronic heart failure: a retrospective cohort study. Heart 2002; 87: 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Minaga T, Takeda K, Nakamura T, Kizu A, Ijichi H. Effect of xanthine oxidase inhibitor on myocardial ischemia. Recent Adv Stud Cardiac Struct Metab 1976; 11: 555–558. [PubMed] [Google Scholar]
- 4. Saavedra WF, Paolocci N, St John ME, Skaf MW, Stewart GC, Xie JS, Harrison RW, Zeichner J, Mudrick D, Marban E, Kass DA, Hare JM. Imbalance between xanthine oxidase and nitric oxide synthase signaling pathways underlies mechanoenergetic uncoupling in the failing heart. Circ Res 2002; 90: 297–304. [DOI] [PubMed] [Google Scholar]
- 5. Ukai T, Cheng CP, Tachibana H, Igawa A, Zhang ZS, Cheng HJ, Little WC. Allopurinol enhances the contractile response to dobutamine and exercise in dogs with pacing induced heart failure. Circulation 2001; 103: 750–755. [DOI] [PubMed] [Google Scholar]
- 6. Johnson WD, Kayser KL, Brenowitz JB, Saedi SF. A randomized controlled trial of allopurinol in coronary bypass surgery. Am Heart J 1991; 121: 20–24. [DOI] [PubMed] [Google Scholar]
- 7. Ekelund UE, Harrison RW, Shokek O, Thakkar RN, Tunin RS, Senzaki H, Kass DA, Marban E, Hare JM. Intravenous allopurinol decreases myocardial oxygen consumption and increases mechanical efficiency in dogs with pacing induced heart failure. Circ Res 1999; 5: 437–445. [DOI] [PubMed] [Google Scholar]
- 8. Cappola TP, Kass DA, Nelson GS, Berger RD, Rosas GO, Kobeissi ZA, Marban E, Hare JM. Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation 2001; 20: 2407–2411. [DOI] [PubMed] [Google Scholar]
- 9. Amado LC, Saliaris AP, Raju SV, Lehrke S, St John M, Xie J, Stewart G, Fitton T, Minhas KM, Brawn J, Hare JM. Xanthine oxidase inhibition ameliorates cardiovascular dysfunction in dogs with pacing‐induced heart failure. J Mol Cell Cardiol 2005; 3: 531–536. [DOI] [PubMed] [Google Scholar]
- 10. Engberding N, Spiekermann S, Schaefer A, Heineke A, Wiencke A, Muller M, Fuchs M, Hilfiker‐Kleiner D, Hornig B, Drexler H, Landmesser U. Allopurinol attenuates left ventricular remodeling and dysfunction after experimental myocardial infarction: a new action for an old drug? Circulation 2004; 15: 2175–2179. [DOI] [PubMed] [Google Scholar]
- 11. Naumova AV, Chacko VP, Ouwerkerk R, Stull L, Marban E, Weiss RG. Xanthine oxidase inhibitors improve energetics and function after infarction in failing mouse hearts. Am J Physiol Heart Circ Physiol 2006; 2: H837–H843. [DOI] [PubMed] [Google Scholar]
- 12. Minhas KM, Saraiva RM, Schuleri KH, Lehrke S, Zheng M, Saliaris AP, Berry CE, Barouch LA, Vandegaer KM, Li D, Hare JM. Xanthine oxidoreductase inhibition causes reverse remodeling in rats with dilated cardiomyopathy. Circ Res 2006; 2: 271–279. [DOI] [PubMed] [Google Scholar]
- 13. Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008; 359: 1811–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anker SD, Doehner W, Rauchhaus M, Sharma R, Francis D, Knosalla C, Davos CH, Cicoira M, Shamim W, Kemp M, Segal R, Osterziel KJ, Leyva F, Hetzer R, Ponikowski P, Coats AJ. Uric acid and survival in chronic heart failure: validation and application in metabolic, functional, and hemodynamic staging. Circulation 2003; 107: 1991–1997. [DOI] [PubMed] [Google Scholar]
- 15. Anker SD, Leyva F, Poole‐Wilson PA, Kox WJ, Stevenson JC, Coats AJ. Relation between serum uric acid and lower limb blood flow in patients with chronic heart failure. Heart 1997; 78: 39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reyes AJ. The increase in serum uric acid concentration caused by diuretics might be beneficial in heart failure. Eur J Heart Fail 2005; 7: 461–467. [DOI] [PubMed] [Google Scholar]
- 17. Higgins P, Dawson J, Lees KR, McArthur K, Quinn TJ, Walters MR. Xanthine oxidase inhibition for the treatment of cardiovascular disease: a systematic review and meta‐analysis. Cardiovasc Ther 2012; 30: 217–226. [DOI] [PubMed] [Google Scholar]
- 18. Zhang J, Dierckx R, Cleland JG. Xanthine oxidase inhibition for the treatment of cardiovascular disease: a systematic review and meta‐analysis. Cardiovascular Therapeutics 2014; 32: 57–58. [DOI] [PubMed] [Google Scholar]
- 19. Givertz MM, Anstrom KJ, Redfield MM, Deswal A, Haddad H, Butler J, Tang WHW, Dunlap ME, LeWinter MM, Mann DL, Felker GM, O'Connor CM, Goldsmith SR, Ofili EO, Saltzberg MT, Margulies KB, Cappola TP, Konstam MA, Semigran MJ, McNulty SE, Lee KL, Shah MR, Hernandez AF. NHLBI. Heart failure clinical research network effects of xanthine oxidase inhibition in hyperuricemic heart failure patients: The Xanthine Oxidase Inhibition for Hyperuricemic Heart Failure Patients (EXACT‐HF) Study. Circulation 2015; 131: 1763–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taheraghdam AA, Sharifipour E, Pashapour A, Namdar S, Hatami A, Houshmandzad S, Sadeghihokmabadi E, Tazik M, Rikhtegar R, Mahmoodpoor A. Allopurinol as a preventive contrivance after acute ischemic stroke in patients with a high level of serum uric acid: a randomized, controlled trial. Medical principles and practice: international journal of the Kuwait University. Health Science Centre 2014; 23: 134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. OpenMed. 2009; 3: e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 22. Rentoukas E, Tsarouhas K, Tsitsimpikou C, Lazaros G, Deftereos S, Vavetsi S. The prognostic impact of allopurinol in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention. Int J Cardiol 2010; 145: 257–258. [DOI] [PubMed] [Google Scholar]
- 23. Coghlan JG, Flitter WD, Clutton SM, Panda R, Daly R, Wright G, IIsley CD, Slater TF. Allopurinol pretreatment improves postoperative recovery and reduces lipid peroxidation in patients undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg 1994; 107: 248–256. [PubMed] [Google Scholar]
- 24. Talwar S, Sandeep JA, Choudhary SK, Velayoudham D, Lakshmy R, Kasthuri JM, Kumar AS. Effect of preoperative administration of allopurinol in patients undergoing surgery for valvular heart diseases. Eur J Cardiothorac Surg 2010; 38: 86–90. [DOI] [PubMed] [Google Scholar]
- 25. Rentoukas E, Tsarouhas K, Tsitsimpikou C, Lazaros G, Deftereos S, Vavetsi S. The prognostic impact of allopurinol in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention. Int J Cardiol 2010; 145: 257–258. [DOI] [PubMed] [Google Scholar]
- 26. Gavin AD, Struthers AD. Allopurinol reduces B‐type natriuretic peptide concentrations and haemoglobin but does not alter exercise capacity in chronic heart failure. Heart 2005; 91: 749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hare JM, Mangal B, Brown J, Fisher CJ, Freudenberger R, Colucci WS, Mann DL, Liu P, Givertz MM, Schwarz RP. Impact of oxypurinol in patients with symptomatic heart failure—results of the OPT‐CHF Study. J Am Coll Cardiol 2008; 51: 2301–2309. [DOI] [PubMed] [Google Scholar]
- 28. Di Stolfo G, Mastroianno S, Potenza DR, De Luca G, d'Arienzo C, Pacilli MA, Fanelli M, Russo A, Fanelli R. Serum uric acid as a prognostic marker in the setting of advanced vascular disease: a prospective study in the elderly. J Geriatr Cardiol 2015; 12: 515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. von Lueder TG, Girerd N, Atar D, Agewall S, Lamiral Z, Kanbay M, Pitt B, Dickstein K, Zannad F, Rossignol P. High‐Risk Myocardial Infarction Database Initiative Investigators. Serum uric acid is associated with mortality and heart failure hospitalizations in patients with complicated myocardial infarction: findings from the High‐Risk Myocardial Infarction Database Initiative. Eur J Heart Fail 2015; 17: 1144–1151. [DOI] [PubMed] [Google Scholar]
- 30. Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 1999; 131: 7–13. [DOI] [PubMed] [Google Scholar]
- 31. Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006; 58: 87–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Santos CX, Anjos EI, Augusto O. Uric acid oxidation by peroxynitrite: multiple reactions, free radical formation, and amplification of lipid oxidation. Arch Biochem Biophys. 1999; 372: 285–294. [DOI] [PubMed] [Google Scholar]
- 33. Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000; 87: 840–844. [DOI] [PubMed] [Google Scholar]
- 34. Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, Krotova K, Block ER, Prabhakar S, Johnson RJ. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005; 67: 1739–1742. [DOI] [PubMed] [Google Scholar]
- 35. Corry DB, Eslami P, Yamamoto K, Nyby MD, Makino H, Tuck ML. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin–angiotensin system. J Hypertens. 2008; 26: 269–275. [DOI] [PubMed] [Google Scholar]
- 36. Filippatos GS, Ahmed MI, Gladden JD, Mujib M, Aban IB, Love TE, Sanders PW, Pitt B, Anker SD, Ahmed A. Hyperuricaemia, chronic kidney disease, and outcomes in heart failure: potential mechanistic insights from epidemiological data. Eur Heart J 2011; 32: 712–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ekundayo OJ, Dell'Italia LJ, Sanders PW, Arnett D, Aban I, Love TE, Filippatos G, Anker SD, Lloyd‐Jones DM, Bakris G, Mujib M, Ahmed A. Association between hyperuricemia and incident heart failure among older adults: a propensity‐matched study. Int J Cardiol 2010; 142: 279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Struthers AD, Donnan PT, Lindsay P, McNaughton D, Broomhall J, MacDonald TM. Effect of allopurinol on mortality and hospitalisations in chronic heart failure: a retrospective cohort study. Heart 2002; 87: 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wei L, Fahey T, Struthers AD, MacDonald TM. Association between allopurinol and mortality in heart failure patients: a long‐term follow‐up study. Int J Clin Pract 2009; 63: 1327–1333. [DOI] [PubMed] [Google Scholar]
- 40. Raval J, Nagaraja V, Eslick GD, Denniss AR. The role of colchicine in pericarditis—a systematic review and meta‐analysis of randomised trials. Heart Lung Circ 2015; 24: 660–666. [DOI] [PubMed] [Google Scholar]
- 41. Solomon DH, Liu CC, Kuo IH, Zak A, Kim SC. Effects of colchicine on risk of cardiovascular events and mortality among patients with gout: a cohort study using electronic medical records linked with medicare claims. Ann Rheum Dis 2016; 75: 1674–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goicoechea M, de Vinuesa SG, Verdalles U, Ruiz‐Caro C, Ampuero J, Rincon A, Arroyo D, Luno J. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol 2010; 5: 1388–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Egger M, Davey SG, Schncider M, Minder CE. Bias in meta‐analysis detected by a simple graphical test. British Medical Journal 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Villar J, Piaggio G, Carroli G, Donner A. Factors affecting the comparability of meta‐analyses and largest trials results in perinatology. Journal of Clinical Epidemiology 1997; 50: 997–1002. [DOI] [PubMed] [Google Scholar]


