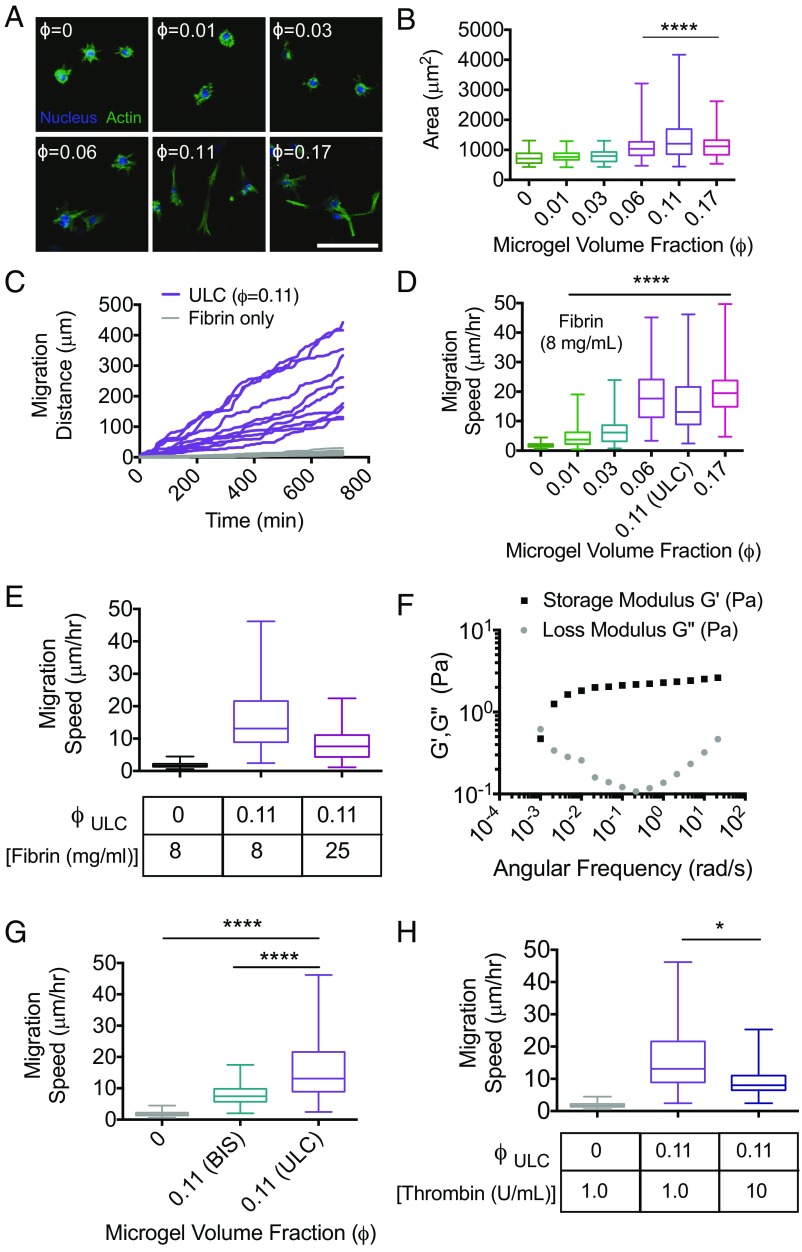
Fig. 4.
μGel-filled, percolated colloidal assemblies enable enhanced cell spreading and invasion of dense, nonpermissive fibrin polymers. Fibroblast morphology and motility were analyzed in a variety of fibrin–μgel formulations (ULC μgels unless otherwise noted). (A) Representative maximum intensity projections of confocal image slices of the actin cytoskeleton and nuclei of fibroblasts embedded in fibrin (8 mg/mL) with increasing ϕ. (Scale bar, 100 μm.) These images indicate that fibroblasts do not appreciably spread until ϕ approaches ϕc. (B) Quantitation of cell spread area indicates maximum spreading is observed at ϕc. A partial transition to a spread state is observed at ϕ = 0.06, before full network percolation, whereas cells also appear less well spread at ϕ that far exceeds the critical threshold for percolation. (C) Representative cumulative distance vs. time for cell migration in formulations at ϕc demonstrate cumulative distances that span the overall dimensions of the colloidal network. (D) Average migration speed was measured at increasing ϕ. In correlation with spread area, cell migration speeds increase significantly as ϕ approaches ϕc. (E) Average migration speeds of fibroblasts decrease in formulations made with 25 mg/mL fibrin, compared with 8 mg/mL, but remained higher than fibrin-only formulations at lower concentrations (8 mg/mL). Whereas elastic deformation of individual μgels cannot account for the motility observed, cells could use the long-time viscous behavior of the μgel suspension, thus (F) μgel suspensions at ϕfinal = 0.6 were tested with rheology to measure G′, G′′ as a function of angular frequency to determine the timescale of their structural relaxation. Furthermore, (G) cell migration speed as a function of μgel cross-linking density and (H) thrombin concentrations were measured. All data are represented as box and whiskers plots (25th to 75th percentile) with a line at the median and error bars to the minimum and maximum values. A Kruskal–Wallis nonparametric test with Dunn’s multiple comparisons posttest was used for B, D–G. *P < 0.05, ****P < 0.0001. Cells could phagocytize some μgels to initially enter the tunnel structure although there is no evidence for phagocytosis in our experiments. Subsequent phagocytosis assays show no evidence of cell uptake. The long migration distances also suggest that the cells rather exploit the long-time viscous behavior of the μgel suspension.