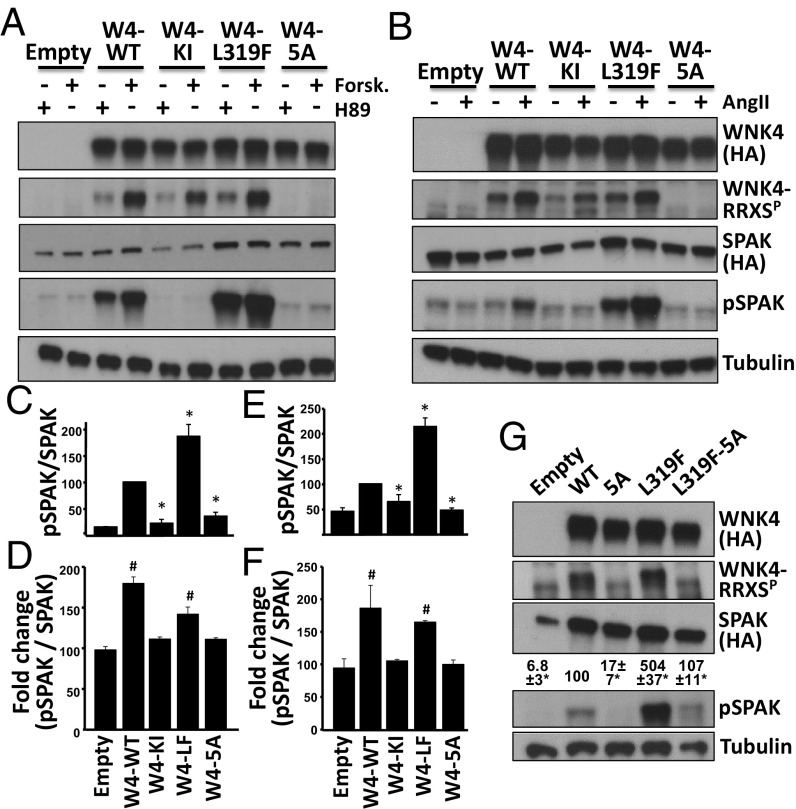
Fig. 4.
Functional relevance of WNK4 phosphorylation in RRXS sites. (A) COS-7 cells were transfected with SPAK-HA and WNK4-HA or the indicated WNK4 mutants and stimulated with H89 (20 μM) or forskolin (30 μM). Lysates were blotted with the indicated antibodies. (B) HEK293T cells were transfected with SPAK-HA, ATI receptor and WNK4-HA, or the indicated mutants. After overnight serum depletion, cells were stimulated with vehicle or AngII (100 nM). Lysates were blotted with the indicated antibodies. KI, kinase inactive; 5A, mutant in which the Ser residues of the five RRXS motifs were substituted for Ala. (C) Quantitation of pSPAK/SPAK for H89-treated groups represented in A. (D) Fold-change in pSPAK/SPAK between H89 and forskolin groups. (E) Quantitation of pSPAK/SPAK for AngII-treated groups represented in B. (F) Fold-change in pSPAK/SPAK between vehicle and AngII groups. All data are means ± SEM; n ≥ 3. *P < 0.05 vs. WT; #P < 0.05 vs. Empty. Results of quantitation for RRXSP and SPAK blots are shown in Fig. S3. (G) Cells were transfected with SPAK and different WNK4 mutants. Note that L319F-5A makes reference to a single clone that presents six mutations. SPAK phosphorylation was assessed. Results of quantitation are shown above pSPAK blot. All data are means ± SEM; n = 3. See also Figs. S3 and S4.