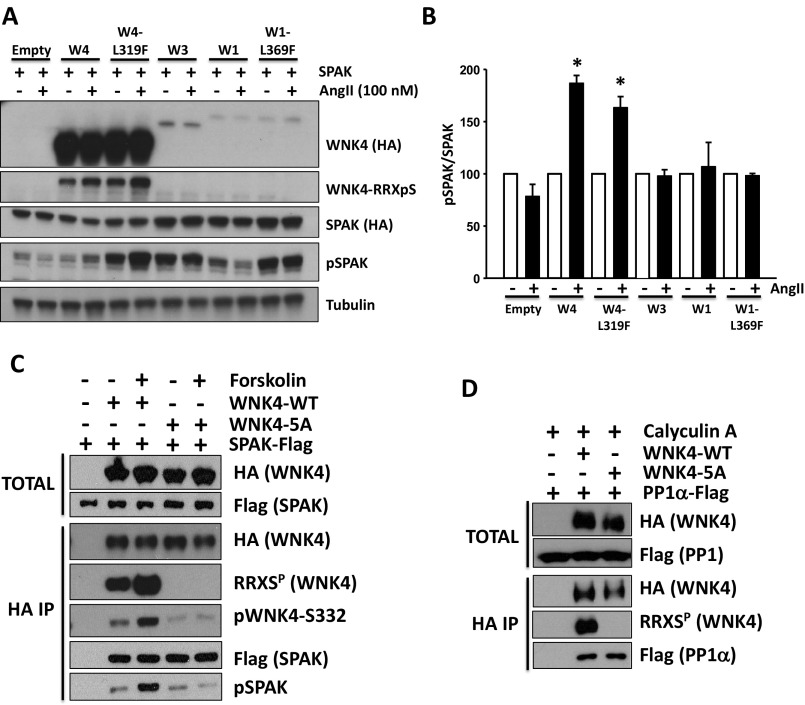
Fig. S4.
AngII-induced phosphorylation of SPAK is exclusively mediated by WNK4. (A) HEK293T cells were transfected with SPAK-HA, ATI receptor and the indicated WNK kinase. At 48 h posttransfection, cells were stimulated with 100 nM AngII for 30 min and then lysed. Protein extracts were immunoblotted with the indicated antibodies. The increase in pSPAK signal promoted by AngII stimulation was only observed in the presence of WNK4 or WNK4-L319F. (B) Band intensities from the pSPAK blots from three different experiments were quantified and the results are represented in the graph as means ± SEM. Values were normalized, establishing the vehicle groups as 100%. *P < 0.05 vs. Veh. (C) SPAK binding to WNK4 is not affected by WNK4–RRXS phosphorylation. HEK293T cells were transfected with SPAK-FLAG and wild-type WNK4-HA or WNK4-5A-HA. After overnight serum depletion, the indicated groups of cells were treated with forskolin to promote WNK4-RRXS phosphorylation. WNK4 was immunoprecipitated using Sepharose beads bound to anti-HA antibody and levels of coimmunoprecipitated SPAK were analyzed by Western blot. Three independent experiments were performed with similar results. (D) PP1α binding to WNK4 is not affected by WNK4–RRXS phosphorylation. HEK293T cells were transfected with PP1α-FLAG and wild-type WNK4-HA or WNK4-5A-HA. All groups were treated with calyculin A for 20 min before lysis to prevent RRXS dephosphorylation by PP1α. Three independent experiments were performed with similar results.