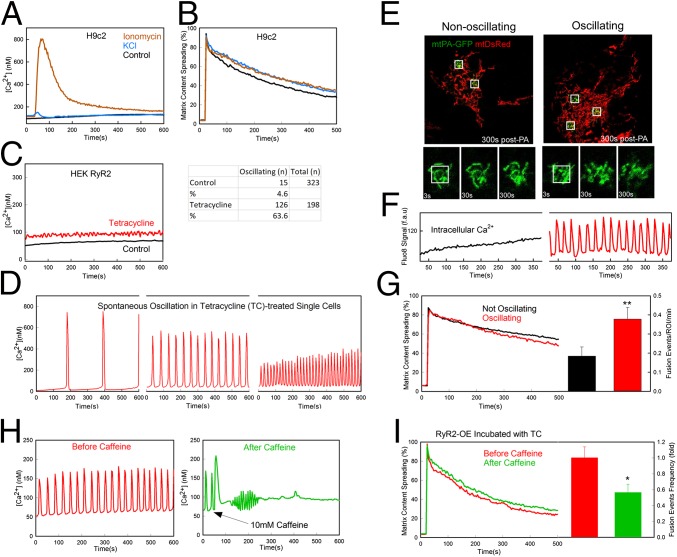
Fig. 7.
Effect of calcium oscillations and plasma membrane depolarization on mitochondrial dynamics in H9c2 and HEK cells. (A) Fura2-AM–loaded H9c2 cells were treated with 60 mM KCl (n = 179), treated with 5 µM ionomycin (n = 159), or left untreated (control; n = 348) at 30 s. [Ca2+]c was recorded every 3 s for 10 min. (B) The change in mtPA-GFP/mtDsRed is shown for H9c2 cells treated with 60 mM KCl (n = 31), treated with 5 µM ionomycin (n = 27), or untreated from 5 min before imaging (n = 36 cells). (C, Left) HEK-RyR2 cells induced with tetracycline show increased mean basal [Ca2+]c compared with WT because of spontaneous oscillations. (C, Right) Percentage of cells in each group showing spontaneous oscillation. (D) Samples of HEK-RyR2 cells showing spontaneous calcium oscillation measured with Fluo8-AM show greater amplitude when the oscillations occur at a lower frequency. (E) Spreading of mtPA-GFP and recovery of mtDsRed in nonoscillating and oscillating HEK-RyR2 cells (Upper) and time series of one PA region from the cells in the upper panel (Lower). (F) [Ca2+]c recorded with Fluo8 (fluorescence arbitrary units) in the cells shown in E. (G) Comparison of mtPA-GFP spreading between oscillating and nonoscillating HEK-RyR2 cells. Mean spreading rates show little difference (Left); however, the oscillating cells (n = 30) show roughly a twofold increase in fusion events per PA region compared with the nonoscillating cells (n = 34) (Right). **P < 0.01. (H) [Ca2+]c in a single oscillating HEK-RyR2 cell before and after treatment with 10 mM caffeine (n = 314 and 297, respectively). (I) Comparison of GFP diffusion and relative rates of fusion frequency in HEK-RyR2 cells before and after caffeine treatment (n = 12 and 13, respectively, for PA-GFP diffusion; n = 6 and 7, respectively, for mitochondrial fusion; P < 0.05).