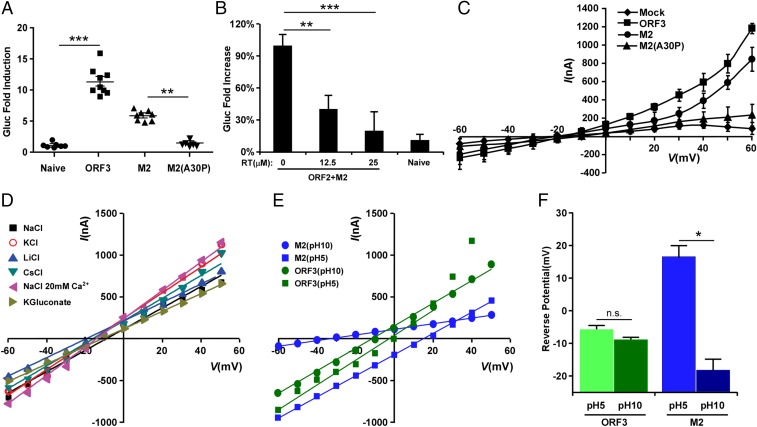
Fig. 3.
The HEV ORF3 protein displays ion channel activity. (A) IAV M2 can substitute ORF3 function. Cell culture supernatants from naïve HepG2C3A, or HepG2C3A cells transduced with HEV ORF2 and ORF3, M2 or M2(A30P), were collected 5 d posttransfection with rHEVΔORF2/3[Gluc] RNA. Naïve HepG2C3A cells were incubated with these supernatants. After 12 h, cells were washed and Gaussia luciferase activity quantified in the cell culture supernatants 4 d later. Data represent the mean ± SD (n = 6–9). *P < 0.05, **P < 0.01, and ***P < 0.001. (B) Inhibition of IAV M2 function with rimantadine (Sigma-Aldrich) suppresses release of HEV particles. Cell supernatants from naïve HepG2C3A or HepG2C3A cells transduced with HEV ORF2 and IAV M2 in the presence of indicated concentrations of rimantadine, were collected 5 d posttransfection with rHEVΔORF2/3[Gluc] RNA. Naïve HepG2C3A cells were incubated with these supernatants. After 12 h, cells were washed and Gaussia luciferase activity quantified in the cell culture supernatants 4 d later. Results are presented as percentage relative to the carrier-treated control. Data represent the mean ± SD *P < 0.05, **P < 0.01, and ***P < 0.001. (C) Current–voltage relationship of X. laevis oocytes expressing HEV ORF3, IAV M2, IAV M2(A30P) or control oocytes. During the current recording, the oocytes were bathed in Ringer solution. The standard voltage-clamp protocol consisted of rectangular voltage step pulses from −90 to +60 mV in 10-mV increments applied from a holding voltage of −60 mV. Each point represents the steady-state current (average current between 4,000–5,000 ms) at the corresponding voltage step. Data represent the mean ± SD (n = 5). (D) ORF3 has little to no ion selectivity. Shown is the current–voltage relationship of the peak tail currents of HEV ORF3 at different ionic conditions. To assess monovalent cation selectivity, K+, Cs+, or Li+ replaced Na+ at equimolar concentration. These substitutions did not alter the reversal potential of the HEV ORF3 currents. In addition, no significant change in reversal potential was observed when 20 mM CaCl2 was added to the solution or when Cl− was replaced by gluconate. (E) ORF3 channels, unlike M2, are nonselective to protons. Current–voltage relationship of the instantaneous current of HEV ORF3 (green) and M2 (blue). To assess for proton permeability oocytes expressing M2 or ORF3 channels were exposed to bath solutions at pH 10 and 5 (Experimental Procedures). Oocytes were clamped at −80 mV, followed by a 1-s depolarization to +20 mV. The voltage was then stepped to test voltages ranging from −80 to +50 mV in 10-mV increments. (F) Graph shows average reversal potential (Erev) for HEV ORF3 (green) and M2 (blue) channels. Error bars show SEM (n = 7). A one-way ANOVA confirmed difference between the different groups (F(3, 27) = 34.187, P < 0.005). Tukey’s honest significant difference (HSD) tests revealed significant differences between the M2 at pH 5 and M2 at pH 10 (P < 0.004) groups. This shift of Erev for M2 at different pHs support proton selectivity; in contrast, no difference was found for HEV ORF3.