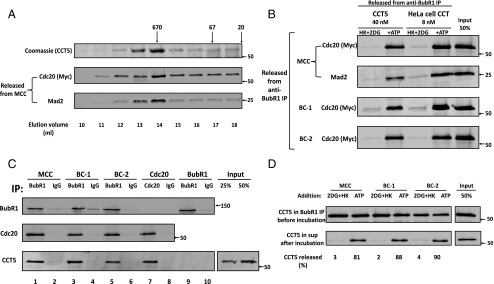
Fig. 3.
Activity and properties of recombinant CCT5 chaperonin complex in MCC disassembly. (A) Separation of recombinant CCT5 homooligomer by gel filtration chromatography. The pET21b plasmid-containing human his6–CCT5 gene insert (generously provided by Jonathan A. King, Massachusetts Institute of Technology, Cambridge, MA) was transformed into BL21 (DE3), and was expressed and purified on Ni-NTA agarose, as described (23). This material was applied to a Superose 6 10/300 GL size exclusion column (GE Healthcare) equilibrated with 20 mm Hepes/KOH, pH 7.4, 300 mm NaCl, 10 mm MgCl2, 5% (vol/vol) glycerol, and 1 mM DTT. Fractions of 0.5 mL were collected at a flow rate of 0.5 mL/min. Samples of 10 μL of column fractions at the indicated elution volumes were subjected to SDS/PAGE and Coomassie staining. Samples of 3 µL of the indicated fractions were analyzed for MCC disassembly as described in Materials and Methods, and released material was immunoblotted for myc–Cdc20 and Mad2. Arrows at the Top indicate the elution position of molecular mass marker proteins (in kilodaltons). (B) Comparison of dissociation of MCC, BC-1, and BC-2 by recombinant CCT5 complex and by purified HeLa cell CCT/TRiC chaperonin. Peak fractions of recombinant homooligomeric CCT5 complex and of purified heterooligomeric HeLa cell CCT/TRiC shown in Figs. 3A and 2B, respectively, were pooled and concentrated. The disassembly of various recombinant mitotic complexes by indicated concentrations of these preparations was assayed as described in Materials and Methods. Where indicated, additions were as follows: ATP, 2 mM, together with 10 mM phosphocreatine and 100 μg/mL creatine phosphokinase and HK, 400 μg/mL, together with 10 mM 2DG. Arrows on the Right indicate the positions of marker proteins (in kilodaltons). (C) CCT5 complex binds MCC, its subcomplexes and free Cdc20, but not free BubR1. The indicated mitotic checkpoint complexes were added to the assay at 100 nM in the presence of 100 nM purified CCT5 complex. Reaction mixtures contained in a volume of 25 μL ingredients at concentrations similar to those described for the assay of dissociation of complexes (Materials and Methods), except that ATP was omitted and 2-deoxyglucose and hexokinase were added. Following rotation at 4 °C for 2 h, samples were mixed with 5 μL of the indicated antibody-bound beads and subjected to immunoprecipitation with similar rotation. Immunoprecipitated material was then washed and analyzed as described in Fig. S5. Arrows on the Right indicate the positions of marker proteins (in kilodaltons). (D) Release of CCT5 from mitotic checkpoint complexes is ATP dependent. The indicated mitotic checkpoint complex proteins were first bound to CCT5 complex in the absence of ATP, as described in Fig. 3C. Subsequently, samples were immunoprecipitated with anti-BubR1 beads, beads were washed and then incubated (Materials and Methods) as indicated with hexokinase and 2-deoxyglucose (2DG+HK) or ATP. The release of CCT5 from anti-BubR1 beads was estimated and expressed as the percentage of initial CCT5 bound. Arrows on the Right indicate the positions of marker proteins (in kilodaltons).