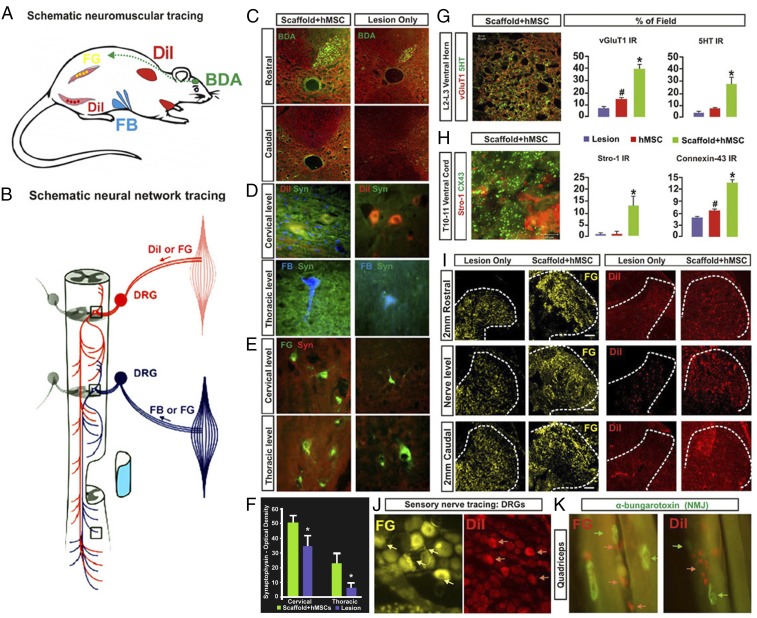
Fig. 6.
Neurobiological benefits resulting from the scaffolded hMSC treatment. (A) Tracing regimens: BDA (green; injected into motosensory cortex contralateral to hemisection) for tracing the CST on the lesioned side, whereas DiI, Fast Blue (FB), and Fluoro-Gold (FG) were injected ipsilaterally into cervical region muscles and intercostal and abdominal muscles (Materials and Methods) to trace both primary proprioceptive axons that synapse with the PSN interneurons and with ventral horn motor neurons. DiI and FG were also injected into hindlimb muscles for retrograde tracing of lumbar motor neurons and primary afferent fibers. (B) Schematic of a simplified PSN network consisting of cross-segmental 1° and 2° neurites that project bilaterally and bidirectionally. (C) BDA IR was detected in the ipsilateral CST rostral to the injury epicenter, but not at any levels caudal to the lesion site in both scaffolded hMSC-treated and lesion-only spinal cords, suggesting no CST regeneration. (D and E) Images of DiI-, FB-, or FG-traced cervical and thoracic ventral horn motor neurons, respectively, and the synapses in the surrounding regions that were costained by antibody against synaptophysin (Syn, green), a synaptic vesicle marker. Scaffolded hMSCs-treated spinal cords showed much higher Syn IR density in both cervical and thoracic cord regions, compared with lesion-only (or hMSCs-only) controls (F; P < 0.05, paired Student’s t test, n = 5 per group). (G, Left) Tissue slices selected from upper lumbar segment 5 mm caudal to the epicenter were immunostained for 5-HT (red) and vGluT1 (green). A representative z-stack confocal plane (20 × 0.1 μm optical sections) of the Rexed Lamina VIII area is shown (Inset) at 600× magnification, and presents typical details of the pericellular distribution of axon terminal vGluT1 and beaded varicosities of 5HT. (Scale bar: 5 µm.) (G, Right) Group average IR of vGluT1 (red) that marks primary proprioceptive terminals and 5HT (green) in lumbar Rexed Lamina VIII were significantly stronger in scaffolded hMSC-treated spinal cords compared with controls. (H) Coimmunostaining of STRO-1 (red), an hMSC marker and Cx43 (green), a gap junction protein, revealed significantly higher signal density of Cx43 in the host ventral cord region of sections immediately caudal to the scaffolded hMSC implant site, compared with the control groups (*, scaffolded hMSC vs. hMSC only and lesion only; #, hMSC only vs. lesion only; P < 0.05, n = 5 per group; one-way ANOVA with Tukey’s post hoc test). Cx43 was expressed mainly by host cells (i.e., STRO-1–negative cells). (I) Retrograde tracing with FG or DiI via T6–T8 intercostal nerve showed FG+ or DiI+ primary afferent fibers and (J) DRG neurons. Scaffolded hMSC treatment drastically increased densities and area of both tracers, relative to the lesion controls, and DRG neuron integrity (J). (K) Motor nerve endings are in close contact with α-bungarotoxin+ nicotinic receptors in the muscles, showing near normal morphology of NMJs in the quadriceps of scaffolded hMSC-treated rats.