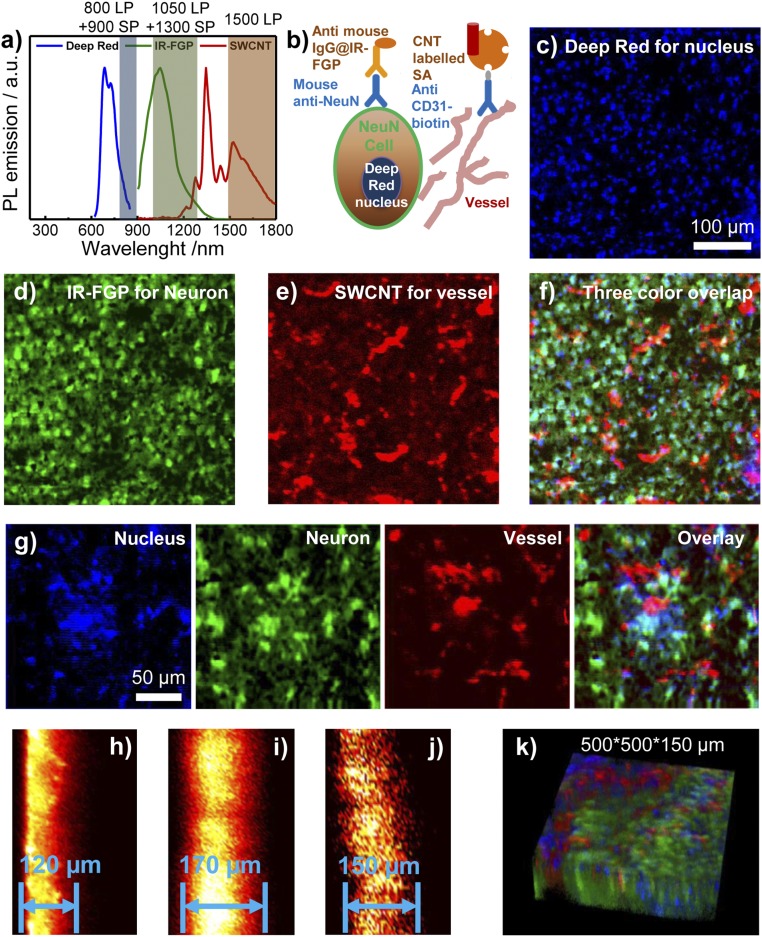
Fig. 3.
Multicolor 2D/3D staining in NIR-I and NIR-II windows (800–1,700 nm). (A) PL emission spectra of Deep Red, IR-FGP, and IIb SWCNT including the long-pass/short-pass filter ranges used for three-color imaging. (Although there is slight emission overlay between IR-FGP and SWCNT, the lower QY of SWCNT did not affect the IR-FGP channel.) (B) Scheme of molecular imaging with three color channels. (C–F) 2D multicolor labeling of brain tissue imaged with a home-built confocal system. (C) Deep Red with NIR-I emission for staining the nucleus (658-nm excitation with 850-nm long-pass and 900-nm short-pass emission filters. Note that the 850-nm long-pass filter can be replaced with an 800-nm filter in the present confocal setup to get similar results with higher signal intensities. Images were plotted by ImageJ, increasing the z-scale to eliminate autofluorescence and NSB background signal. SI Appendix, Fig. S8E provides raw data without low z-scale changes. (D) Mouse anti-neuron and anti-mouse IgG@IR-FGP with NIR-IIa emission for staining the neuron (785-nm excitation with 1,050-nm long-pass and 1,300-nm short-pass emission filters). Clear visualization of neuron nucleus with light staining of cell cytoplasm is evident. (E) Anti-CD31 and SA@SWCNT with NIR-IIb emission for staining vessels (785-nm excitation with 1,500-nm long-pass emission filter). (F) Three-color overlapping image of nucleus, neurons, and vessels. (G) Magnified multicolor brain tissue staining with higher resolution. (H–J) Confocal scanning from cross-sections of brain tissue. Scanning depths: Deep Red (H), 120 µm; IR-FGP (I), 170 µm; SWCNT (J), 150 µm. For cross-sectional scanning, the achievable imaging depth was defined as the depth beyond which the measured S/B ratio fell below 2.5. (K) Three-color 3D rendering of nucleus, neuron, and vessel channels obtained with NIR-I/II confocal microscopy. A homebuilt stage scanning confocal setup was used to obtain confocal images. The 100× objective (Olympus, oil immersion, NA 0.8) focuses the excitation laser to a tiny spot with a few μm diameter onto the sample, and the fluorescence goes through 800-nm long-pass dichoric and emission filters to a photomultiplier tube (PMT) detector. A 150-μm pinhole is used to reject out-of-focus signals. For the Deep Red channel, a 658-nm laser was used for excitation, and the signal was detected with a PMT detector (Hamamatsu H7422-50). For the IR-FGP and SWCNT channels, a 785-nm laser was used for excitation, and the signal was detected with an NIR PMT detector (Hamamatsu H12397-75). The scanning rate was 2.5 ms/pixel.