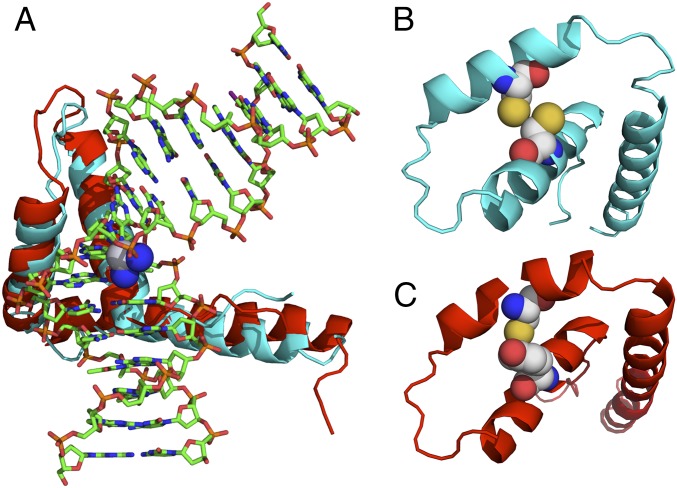
Fig. 1.
Structural representation of the ternary HMGB1/HMGB4–Pt-DNA complex. (A) Schematic representation of the crystal structure of domain A of HMGB1 in complex with platinated DNA superimposed with a computed structure of the similar domain of HMGB4. Significant homology is observed between HMGB4 and HMGB1 domain structures. SWISS-MODEL (46) with input from the human HMGB4 sequence was used to compute the HMGB4 structure. (B) Schematic representation of a simplified structure of HMGB1 protein (ribbon) highlighting Cys22 and Cys44 (space filling), critical residues implicated in disulfide bond formation under oxidizing conditions. (C) Schematic representation of a simplified structure of HMGB4 (ribbon) displaying Tyr22 and Cys44 (space filling). All structures were rendered using Pymol.