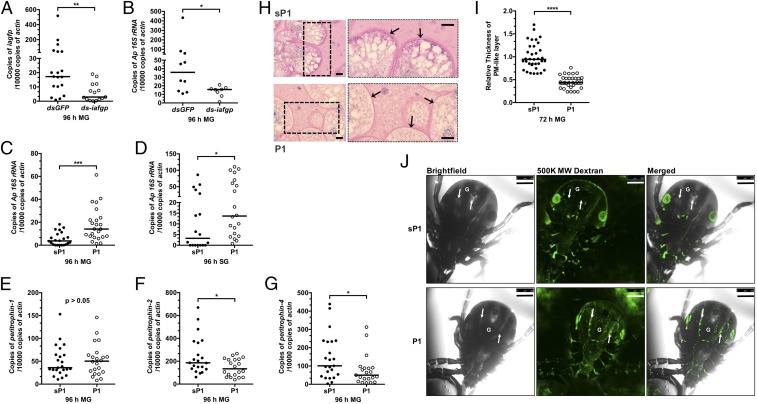
Fig. 3.
IAFGP and its peptide derivative, P1, influence the PM and A. phagocytophilum colonization of tick gut. (A and B) The qRT-PCR examination of the expression of (A) iafgp and (B) the A. phagocytophilum burden in dsgfp- and ds-iafgp-injected nymphs [gut (MG)] fed on A. phagocytophilum-infected mice. Each dot represents one nymph. (C and D) The qRT-PCR assessment of the A. phagocytophilum burden in the (C) guts (MG) and (D) salivary glands with P1- or control [scrambled P1(sP1)]-injected nymphs fed on A. phagocytophilum-infected mice. Each dot represents one nymph. (E−G) The qRT-PCR analysis of expression levels of (E) peritrophin-1, (F) peritrophin-2, and (G) peritophin-4 in P1- and sP1-injected nymphs [gut )MG)]. Each dot represents one nymph. (H) PAS staining of Carnoy’s fixed and sectioned fed guts from P1- and sP1-injected nymphs. Arrows indicate the PAS-positive PM-like layer. Boxed outlines within the images on the left have been magnified 2×. (Scale bar, 10 μm.) (I) Quantification of relative thickness of the PM-like layer from P1- and sP1-injected nymphal guts (MG). Statistical significance was calculated using a two-tailed nonparametric Mann−Whitney test from three pooled experiments (****P < 0.0001; ***P < 0.001; **P < 0.01; *P < 0.05). (J) P1 injection of ticks improves the permeability of the PM. Confocal microscopy of 24-h P1- or sP1-injected nymphs that were capillary-fed Fluorescein-conjugated 500,000 MW dextran (500K MW Dextran). Magnification is 10×. G marks the gut diverticula within the tick. The arrows point to the hemocoel outside the gut. (Scale bar, 250 μm.)