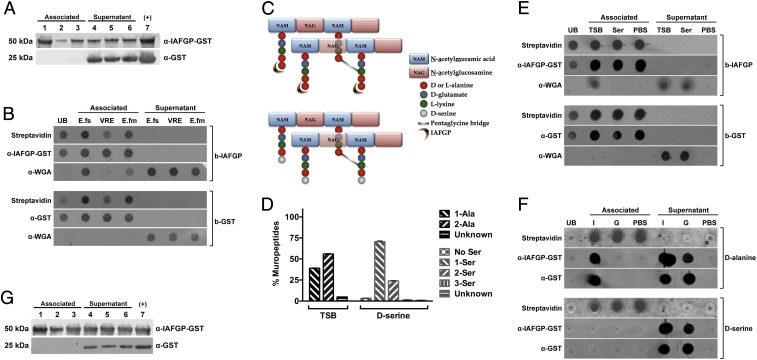
Fig. 5.
IAFGP binds to the terminal d-alanine residue of Enterococcus sp. and S. aureus peptidoglycan. (A) E. faecalis (lanes 1 and 4), E. faecium (lanes 2 and 5), and vancomycin-resistant E. faecalis (lanes 3 and 6) bacteria were incubated with either recombinant GST-IAFGP or GST alone. Bound (Associated) and unbound (Supernatant) protein was detected by immunoblot. Recombinant GST-IAFGP or GST alone (lane 7) was used as a positive control. (B) IAFGP displays varied binding to Enterococcal peptidoglycan. Streptavidin-coated magnetic Dynabeads bound to biotinylated IAFGP-GST (b-IAFGP) or GST alone (b-GST) were incubated with peptidoglycan muropeptides extracted from E. faecalis (E.fs), vancomycin-resistant E. faecalis (VRE), and E. faecium (E.fm) cells grown in BHIG. Biotinylated proteins bound to beads were detected using an infrared-labeled streptavidin probe. IAFGP-GST was detected using a polyclonal murine primary antibody, and GST was detected using a monoclonal murine primary antibody. Muropeptides from cells were detected using a polyclonal WGA antibody. Unbound protein (UB) was also collected and spotted. Associated or Supernatant fractions represent the fractions that were either pulled down with the magnetic bead or remained unbound. (C) Schematic describing S. aureus peptidoglycan and binding site of IAFGP. The S. aureus peptidoglycan backbone is a polymer consisting of glycan strands of alternating sugar subunits: NAG and NAM. The NAM subunits have short peptides (muropeptides) comprising l-alanine, d-glutamate, l-lysine, and two d-alanines. The cross-link between neighboring muropeptides is mediated by a transpeptidase enzyme and allows for the formation of a short peptide interbridge consisting of five glycines (pentaglycine bridge). IAFGP is hypothesized to bind to the terminal d-alanine residue of the stem pentapeptide (Top). Growth of S. aureus in an alternative amino acid like d-serine (Bottom) replaces the terminal d-alanine residue with the newly supplied d-serine. (D) Muropeptide composition of S. aureus peptidoglycan. S. aureus grown in either TSB or medium supplemented with 125 mM d-serine were analyzed for their muropeptide residue composition. Data represent the percentage of total bacterial muropeptides that incorporate a given residue(s) at its carboxyl terminus. “No Ser” represents d-alanine terminating muropeptides. Data were pooled from three independent experiments ± SEM. (E and F) IAFGP binds to the terminal d-alanine residue of the S. aureus muropeptide chain. (E) Streptavidin-coated magnetic Dynabeads bound to b-IAFGP or b-GST were incubated with muropeptides obtained from cells grown in either TSB or medium supplemented with 125 mM d-serine. (F) Experiment was similarly performed as in E using synthetic biotinylated pentapeptides containing either alanine (d-alanine) or serine (d-serine) residues bound to beads and incubated with IAFGP-GST (I) or GST alone (G). PBS was used as a negative binding control. Associated or Supernatant fractions represent the fractions that were either pulled down with the magnetic bead or remained unbound. (G) IAFGP does not bind WTA. Wildtype S. aureus strain SA113 (lanes 1 and 3) and its isogenic WTA-deficient mutant SA113 ΔtagO (lanes 2 and 5) and complemented strain SA113 pRBtagO (lanes 3 and 7) were tested for binding to recombinant GST-IAFGP or control GST alone. Bound (Associated) and unbound (Supernatant) protein was detected by immunoblot analysis. Recombinant GST-IAFGP or GST alone (lane 7) without any bacteria was used as a positive control.