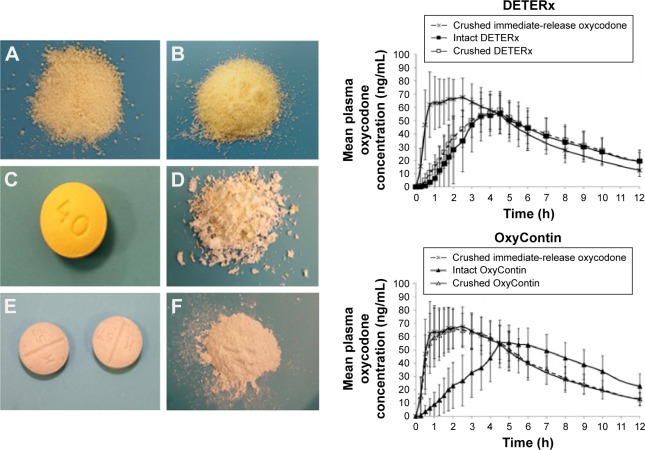
Figure 2.
Left side: (A) intact microspheres (40 mg) obtained by empting one capsule of Deterx®; (B) crushed microspheres (40 mg) from one capsule of Deterx; (C) OxyContin® tablet intact (40 mg); (D) Oxycontin tablet crushed (40 mg); (E) two 20 mg intact immediate-release oxycodone tablets; (F) powder from two crushed immediate-release oxycodone tablets. Right side: mean plasma concentration–time curve profiles generated by the administration of intact and crushed Deterx compared with crushed immediate-release oxycodone (upper figure); mean plasma concentration–time curve profiles generated by the administration of intact and crushed Oxycontin compared with crushed immediate-release oxycodone (lower figure). Reproduced from Gudin J, Levy-Cooperman N, Kopecky EA, Fleming AB. Comparing the effect of tampering on the oral pharmacokinetic profiles of two extended-release oxycodone formulations with abuse-deterrent properties. Pain Med. 2015;16(11):2142–2151, by permission of Oxford University Press.56