ABSTRACT
FMRP is an RNA-binding protein involved in synaptic translation. Its absence causes a form of intellectual disability, the Fragile X syndrome (FXS). Small neuroanatomical abnormalities, present both in human and mouse FMRP-deficient brains, suggest a subtle critical role of this protein in neurogenesis. Stable depletion of FMRP has been obtained in a mouse embryonic stem cell line Fmr1 (shFmr1 ES) that does not display morphological alterations, but an abnormal expression of a subset of genes mainly involved in neuronal differentiation and maturation. Inducing the differentiation of shFmr1 ES cells into the neuronal lineage results in an accelerated generation of neural progenitors and neurons during the first steps of neurogenesis. This transient phenotype is due to an elevated level of the Amyloid Precursor Protein (APP), whose mRNA is a target of FMRP. APP is processed by the BACE-1 enzyme, producing the β-amyloid (Aβ) peptide accelerating neurogenesis by activating the expression of Ascll. Inhibition of the BACE-1 enzyme rescues the phenotype of shFmr1 ES cells.
Here we discuss the importance of the shFmr1 ES line not only to understand the physiopathology of FXS but also as a tool to screen biomolecules for new FXS therapies.
KEYWORDS: cell model for Fragile X syndrome, Fragile X Syndrome, neurogenesis, therapy for Fragile X syndrome
Silencing of the Fragile X Mental Retardation gene (FMR1) causes the Fragile X Syndrome (FXS), the most common form of inherited intellectual disability (ID). FMR1 encodes the Fragile X mental retardation protein (FMRP), an RNA-binding protein involved in different steps of RNA metabolism, such as translational control, RNA transport along neurites and RNA export from the nucleus to the cytoplasm.1 All FXS patients are affected by cognitive impairment and they may display attention deficit-hyperactivity disorder (ADHD), autistic behavior, seizures, anxiety and language delay.2 Examination of brains from FXS patients has shown an increased density of long and tortuous dendritic spines. This abnormality is considered the cellular alteration underpinning FXS ID.3 The Fmr1 null mouse exhibits a phenotype with similarities to humans including abnormal dendrite morphology.4,5 In mice, it has been possible to associate the altered dendritic spine morphology to some abnormal forms of synaptic plasticity (e.g., increased hippocampal LTD, reduced cortical LTP and epileptogenesis).6,7 The functional defects of these neuronal structures characterizing Fmr1-null neurons have been linked to the role of FMRP in the regulation of translation of a subset of synaptic proteins (1,7; see below). In addition, children with FXS (aged between 18 and 42 months) have larger brain volumes and display enlargement in the temporal lobe white matter, cerebellar gray matter and caudate nucleus, but have a smaller amygdala.8 In another study analyzing boys aged 1 to 3 y the authors found, as in the adult patients, increased caudate, fusiform gyrus, and thalamus gray matter volume (GMV) as well as reduced superior temporal gyrus, hippocampus, and orbitofrontal cortex GMV. Specifically, a reduced GMV in the hypothalamus, insula, and medial and lateral prefrontal cortices was also described.9 In C57BL6/J Fmr1-null mice, differences in brain volumes were only observed in two deep cerebellar nuclei (fastigial nucleus and nucleus interpositus).10 On the other hand, FVB Fmr1 knockout mice display different brain phenotypes compared with the same model in the C57B6/J background. In fact, in FVB Fmr1 knockout mice significantly larger relative volume differences were found in major white matter structures throughout the brain. Moreover, a smaller striatum and a larger parieto-temporal lobe volume were observed.11 These neuroanatomical abnormalities are likely to be generated early during development and may be associated to defects in proliferation and/or differentiation of neural progenitors, suggesting a critical role of FMRP in neurogenesis.12
Recent studies on the Down Syndrome, another form of ID, showed the possibility to treat young adults,13 but it was also underlined the importance to start treatments as early as possible. Consequently, some preclinical therapeutic approaches are targeting not only neonatal but also prenatal life14 focusing on those molecules that can act on neurogenesis defects.15,16 Starting from these considerations and from the fact that an effective specific therapy for FXS is not available yet, a mouse embryonic stem cell line displaying a reduced expression of Fmr1 by stable transfection of a specific shRNA directed against Fmr1 (shFmr1 ES) has been generated.17 These cells do not display morphological abnormalities and cell cycle variations, however altered expression of a subset of genes mainly involved in neuronal differentiation and maturation determines a subjacent molecular pathology. Indeed, stimulating the differentiation of shFmr1 ES cells into the neuronal lineage results in an accelerated generation of neural progenitors and neurons during the first steps of differentiation. This phenotype is transient, as the final number of neurons is not affected at late phases of in vitro neurogenesis. Interestingly, neurogenesis is also accelerated in the embryonic brains of Fmr1 KO mice, indicating that the shFmr1 ES cell model recapitulates the molecular and cellular alterations present in vivo.17 This phenotype in shFmr1 ES cells is likely due to an elevated level of the Amyloid Precursor Protein (APP), whose mRNA is a known target of FMRP.18 APP is processed by the BACE-1 enzyme, producing the β-amyloid (Aβ) peptide that is known to accelerate neurogenesis by activating the expression of achaete-scute family bHLH transcription factor 1 (Ascll).19,20 It is interesting to point out that the increased level of Aβ peptide in Fmr1-depleted ES cells induces the expression of Ascll.17 This latter factor has a pivotal role in neuronal differentiation21 and its induction in shFmr1 ES cells represents a surprising event and the key point to explain the subsequent altered neuronal differentiation.17 Consistently, the cell phenotype is rescued not only by re-expressing human FMR1, but also by reducing the processing of APP by the specific BACE-1 inhibitor LY2811376. The importance of the Aβ peptide in the physiopathology of FXS, as well as in other forms of autism and ID, has been extensively studied.22
ShFmr1 ES cells also present altered expression of other genes that could explain the FXS pathology at the molecular level. Indeed, a reduced expression of Tropomyosin Receptor Kinase B (TrkB) is present. Interestingly, treatment of adult Fmr1 knockout mice with 7, 8-dihydroxyflavone (7, 8-DHF), an agonist of TrkB, improves their spatial and fear memory.23 Furthermore, expression of the small GTPase RhoA is reduced in shFmr1 ES cells. The mRNA encoding RhoA was already shown to be a target of FMRP24 and Rho GTPases and actin remodelling have been already described as having a critical role in the physiopathology of FXS.1,24
Collectively, these data underline the fact that the absence of FMRP modulates the expression of proteins and their related pathways spanning the earliest steps of embryonic life to adult. Depletion of FMRP alters the normal kinetics of neuronal differentiation.17 We can speculate that this event uncoordinates different brain maturation pathways and programs, leading to subtle architectural abnormalities of several brain regions and, ultimately, to intellectual deficit.
The phenotype of shFmr1 neural progenitors appears surprising since cell models of neural precursors for genes involved in other forms of ID/autism rather display a delay of neuronal differentiation or a disruption of neurogenesis.25-27 Indeed, premature neurogenesis has been associated to gross brain abnormalities in α thalassemia/mental retardation syndrome X-linked (ATRX), consistently with the microcephaly observed in patients affected by this disorder.28,29 Similarly, accelerated cell cycle and overproduction of GABAergic inhibitory neurons were described in iPSC-derived brain organoids of Autism Spectrum Disorder (ASD) patients characterized by macrocephalia. Molecularly, this abnormal neurogenesis was due to the overexpression of the transcription factor FOXG1.30 However, it is worth noticing that depletion of Phosphatase and TENsin homolog (PTEN) in postnatal/young neural stem cells produced an altered neurogenesis characterized in a first step by an increased proliferation and differentiation rate of these cells.31 However, an early loss of Neural Stem/progenitor Cells (NSCs) was observed in these animals after some months. Interestingly, these mice displayed macrocephaly and, similarly to FXS mice, an impaired social interaction and an activation of AKT, S6 and GSK3b in hippocampal neurons.30 Also in this case, it is possible to describe an altered kinetics of neurogenesis even if, due to the severity of the cellular alterations, the morphological brain abnormality appears more evident than in FXS brains. These considerations underline the importance to study embryonic neurogenesis in ID/autism animal models to decipher the physiopathology of these disorders and to identify helpful biomarkers for translational studies.
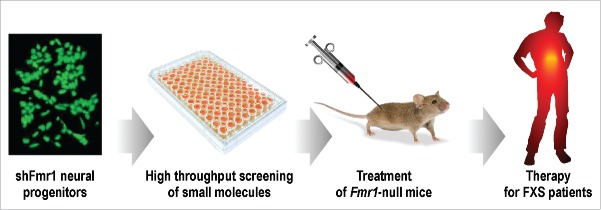
As it has been carefully detailed,17 the genetic heterogeneity of human FXS iPS and FXS human embryonic stem cells17,32-35 complicates the use of these cell lines to study FXS. For this reason, the shFmr1 cell model can be considered as a very useful tool to study neurogenesis in the absence of FMRP.17 In addition, these cells can be very useful to search for novel therapies for FXS. Indeed, they can be used for screening of bioactive molecules, including libraries of small molecules that are already approved for clinical use. This screening can be feasible considering that, as shown above, the phenotype of the FXS cell model can be quantified and is reversible by pharmacological tools. Thus, the molecules that will reveal to be able to actively revert the phenotype of this model could be useful during all developmental ages of patients, since, as we have discussed, some FMRP-dependent pathways are conserved throughout life (Fig. 1). Collectively, these findings will contribute to improve the understanding of the molecular pathology of FXS and to provide a better stratification of FXS patients, which is as a weak aspect in the characterization of this syndrome in view of personalized therapies. New drugs identified by this approach will also highlight new pathways involved in the physiopathology of FXS and specific biomarkers for this syndrome.
Figure 1.
A schema of the different steps of research we plan to carry out to define a treatment starting from the correction of the abnormal morphology of shFmr1 neural progenitors. First, starting from shFmr1 neural progenitors, a high throughput screening will be performed to identify those small molecules (of a selection of already approved drugs) that are able to revert the shFMR1 phenotype. These molecules will be tested in the Fmr1-null mice, the murine model of FXS, to define their ability to rescue some behavioral phenotypes of this mouse at different ages. In particular the impact of these molecules on social interaction and cognition deficits, and in hyperactivity of FXS mice will be evaluated. Molecules able to correct these phenotypes will be directly tested on FXS patients in Phase II clinical trials.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors are grateful to Franck Aguila for graphical work.
Funding
This study was supported by INSERM, CNRS, CNRS LIA NEOGENEX, BB and EL are supported by Agence Nationale de la Recherche: ANR-11-LABX-0028–01. BB is supported by ANR-SVSE4–2012, FRM Team 2014, Monaco Against Autism Foundation, Fondation Jérôme Lejeune.
References
- [1].Maurin T, Zongaro S, Bardoni B. Fragile X Syndrome: from molecular pathology to therapy. Neurosci Biobehav Rev 2014. October; 46 Pt 2:242-55; PMID:24462888; http://dx.doi.org/ 10.1016/j.neubiorev.2014.01.006 [DOI] [PubMed] [Google Scholar]
- [2].Bardoni B, Mandel JL, Fisch GS. FMR1 gene and fragile X syndrome. Am J Med Genet 2000; 97(2):153-63; PMID:11180223; http://dx.doi.org/ 10.1002/1096-8628(200022)97:2%3c153::AID-AJMG7%3e3.0.CO;2-M [DOI] [PubMed] [Google Scholar]
- [3].Ramakers GJA. Rho proteins, mental retardation and the cellular basis of cognition. Trends Neurosci 2002. April; 25(4):191-9; PMID:11998687; http://dx.doi.org/ 10.1016/S0166-2236(00)02118-4 [DOI] [PubMed] [Google Scholar]
- [4].Irwin SA, Galvez R, Greenough WT. Dendritic spine structural anomalies in fragile-X mental retardation syndrome. Cereb Cortex 2000. October; 10(10):1038-44; PMID:11007554; http://dx.doi.org/ 10.1093/cercor/10.10.1038 [DOI] [PubMed] [Google Scholar]
- [5].Grossman AW, Aldridge GM, Weiler IJ, Greenough WT. Local protein synthesis and spine morphogenesis: Fragile X syndrome and beyond. J Neurosci Society for Neuroscience; 2006. July 5; 26(27):7151-5; http://dx.doi.org/ 10.1523/JNEUROSCI.1790-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Catania MV, D apos Antoni S, Bonaccorso CM, Aronica E, Bear MF, Nicoletti F. Group I metabotropic glutamate receptors: a role in neurodevelopmental disorders? Mol Neurobiol 2007. June; 35(3):298-307. [DOI] [PubMed] [Google Scholar]
- [7].D'Antoni S, Spatuzza M, Bonaccorso CM, Musumeci SA, Ciranna L, Nicoletti F, Huber KM, Catania MV. Dysregulation of group-I metabotropic glutamate (mGlu) receptor mediated signalling in disorders associated with Intellectual Disability and Autism. Neurosci Biobehav Rev 2014. October; 46 Pt 2:228-41; PMID:24548786; http://dx.doi.org/ 10.1016/j.neubiorev.2014.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hazlett HC, Poe MD, Lightbody AA, Styner M, MacFall JR, Reiss AL, Piven J. Trajectories of early brain volume development in fragile X syndrome and autism. J Am Acad Child Adolesc Psychiatry 2012. September; 51(9):921-33; PMID:22917205; http://dx.doi.org/ 10.1016/j.jaac.2012.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hoeft F, Carter JC, Lightbody AA, Cody Hazlett H, Piven J, Reiss AL. Region-specific alterations in brain development in one- to three-year-old boys with fragile X syndrome. Proc Natl Acad Sci USA National Acad Sciences 2010. May 18; 107(20):9335-9; http://dx.doi.org/ 10.1073/pnas.1002762107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ellegood J, Pacey LK, Hampson DR, Lerch JP, Henkelman RM. Anatomical phenotyping in a mouse model of fragile X syndrome with magnetic resonance imaging. Neuroimage 2010. November 15; 53(3):1023-9; PMID:20304074; http://dx.doi.org/ 10.1016/j.neuroimage.2010.03.038 [DOI] [PubMed] [Google Scholar]
- [11].Lai JKY, Lerch JP, Doering LC, Foster JA, Ellegood J. Regional brain volumes changes in adult male FMR1-KO mouse on the FVB strain. Neuroscience 2016. March 24; 318:12-21; PMID:26794591; http://dx.doi.org/ 10.1016/j.neuroscience.2016.01.021 [DOI] [PubMed] [Google Scholar]
- [12].Castrén ML. Cortical neurogenesis in fragile X syndrome. Front Biosci (Schol Ed) 2016; 8:160-8; http://dx.doi.org/ 10.2741/s455 [DOI] [PubMed] [Google Scholar]
- [13].Torre la, de R., de Sola S., Hernandez G., Farré M., Pujol J., Rodriguez J, Espadaler JM, Langohr K, Cuenca-Royo A, Principe A, et al.. Safety and efficacy of cognitive training plus epigallocatechin-3 gallate in young adults with Down's syndrome (TESDAD): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Neurol 2016. June; 15(8):801-10; PMID:27302362; http://dx.doi.org/ 10.1016/S1474-4422(16)30034-5 [DOI] [PubMed] [Google Scholar]
- [14].Bartesaghi R, Haydar TF, Delabar JM, Dierssen M, C Martínez-Cué, Bianchi DW. New Perspectives for the Rescue of Cognitive Disability in Down Syndrome. J Neurosci Society for Neuroscience 2015. October 14; 35(41):13843-52; http://dx.doi.org/ 10.1523/JNEUROSCI.2775-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Stagni F, Giacomini A, Emili M, Trazzi S, Guidi S, Sassi M, Ciani E, Rimondini R, Bartesaghi R. Short- and long-term effects of neonatal pharmacotherapy with epigallocatechin-3 gallate on hippocampal development in the Ts65Dn mouse model of Down syndrome. Neuroscience 2016. October 1; 333:277-301; PMID:27457036; http://dx.doi.org/ 10.1016/j.neuroscience.2016.07.031 [DOI] [PubMed] [Google Scholar]
- [16].Giacomini A, Stagni F, Trazzi S, Guidi S, Emili M, Brigham E, Ciani E, Bartesaghi R. Inhibition of APP gamma-secretase restores Sonic Hedgehog signaling and neurogenesis in the Ts65Dn mouse model of Down syndrome. Neurobiol Dis 2015. October; 82:385-96; PMID:26254735; http://dx.doi.org/ 10.1016/j.nbd.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Khalfallah O, Jarjat M, Davidovic L, Nottet N, Cestèle S, Mantegazza M, Bardoni B. Depletion of the fragile X mental retardation protein in embryonic stem cells alters the kinetics of neurogenesis. Stem Cells 2016; [Epub ahead of print]; PMID:27664080; http://dx.doi.org/ 10.1002/stem.2505 [DOI] [PubMed] [Google Scholar]
- [18].Westmark CJ, Malter JS. FMRP mediates mGluR5-dependent translation of amyloid precursor protein Bear MF, editor PLoS Biol. 2007; 5(3):e52; PMID:17298186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Freude KK, Penjwini M, Davis JL, LaFerla FM, Blurton-Jones M. Soluble amyloid precursor protein induces rapid neural differentiation of human embryonic stem cells. J Biol Chem American Society for Biochemistry and Molecular Biology 2011. July 8; 286(27):24264-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Uchida Y, Nakano S-I, Gomi F, Takahashi H. Differential regulation of basic helix-loop-helix factors Mash1 and Olig2 by beta-amyloid accelerates both differentiation and death of cultured neural stem/progenitor cells. J Biol Chem American Society for Biochemistry and Molecular Biology 2007. July 6; 282(27):19700-9. [DOI] [PubMed] [Google Scholar]
- [21].Kim Y, Sung JY, Ceglia I, Lee K-W, Ahn J-H, Halford JM, Kim AM, Kwak SP, Park JB, Ho Ryu S, et al.. Phosphorylation of WAVE1 regulates actin polymerization and dendritic spine morphology. Nature 2006. August 17; 442(7104):814-7; PMID:16862120; http://dx.doi.org/ 10.1038/nature04976 [DOI] [PubMed] [Google Scholar]
- [22].Westmark CJ, Sokol DK, Maloney B, Lahiri DK. Novel roles of amyloid-beta precursor protein metabolites in fragile X syndrome and autism. Mol Psychiatry 2016. October; 21(10):1333-41; PMID:27573877; http://dx.doi.org/ 10.1038/mp.2016.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tian M, Zeng Y, Hu Y, Yuan X, Liu S, Li J, Lu P, Sun Y, Gao L, Fu D, et al.. 7, 8-Dihydroxyflavone induces synapse expression of AMPA GluA1 and ameliorates cognitive and spine abnormalities in a mouse model of fragile X syndrome. Neuropharmacology 2015. February; 89:43-53; PMID:25229717; http://dx.doi.org/ 10.1016/j.neuropharm.2014.09.006 [DOI] [PubMed] [Google Scholar]
- [24].Davidovic L, Navratil V, Bonaccorso CM, Catania MV, Bardoni B, Dumas M-E. A metabolomic and systems biology perspective on the brain of the fragile X syndrome mouse model. Genome Res 2011. December; 21(12):2190-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jolly LA, Homan CC, Jacob R, Barry S, Gecz J. The UPF3B gene, implicated in intellectual disability, autism, ADHD and childhood onset schizophrenia regulates neural progenitor cell behaviour and neuronal outgrowth. Hum Mol Genet Oxford University Press 2013. December 1; 22(23):4673-87; http://dx.doi.org/ 10.1093/hmg/ddt315 [DOI] [PubMed] [Google Scholar]
- [26].Jolly LA, Nguyen LS, Domingo D, Sun Y, Barry S, Hancarova M, Plevova P, Vlckova M, Havlovicova M, Kalscheuer VM, et al.. HCFC1 loss-of-function mutations disrupt neuronal and neural progenitor cells of the developing brain. Hum Mol Genet Oxford University Press; 2015. June 15; 24(12):3335-47; http://dx.doi.org/ 10.1093/hmg/ddv083 [DOI] [PubMed] [Google Scholar]
- [27].Fujitani M, Zhang S, Fujiki R, Fujihara Y, Yamashita T. A chromosome 16p13.11 microduplication causes hyperactivity through dysregulation of miR-484/protocadherin-19 signaling. Mol Psychiatry Nature Publishing Group 2016; PMID:27378146; http://dx.doi.org/ 10.1038/mp.2016.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ritchie K, Watson LA, Davidson B, Jiang Y, NG Bérubé. ATRX is required for maintenance of the neuroprogenitor cell pool in the embryonic mouse brain. Biol Open The Company of Biologists Ltd 2014; 3(12):1158-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Huh MS, Ivanochko D, Hashem LE, Curtin M, Delorme M, Goodall E, Yan K, Picketts DJ. Stalled replication forks within heterochromatin require ATRX for protection. Cell Death Dis Nature Publishing Group 2016; 7(5):e2220; http://dx.doi.org/ 10.1038/cddis.2016.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mariani J, Coppola G, Zhang P, Abyzov A, Provini L, Tomasini L, Amenduni M, Szekely A, Palejev D, Wilson M, et al.. FOXG1-Dependent Dysregulation of GABA/Glutamate Neuron Differentiation in Autism Spectrum Disorders. Cell 2015. July 16; 162(2):375-90; PMID:26186191; http://dx.doi.org/ 10.1016/j.cell.2015.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Amiri A, Cho W, Zhou J, Birnbaum SG, Sinton CM, McKay RM, Parada LF. Pten deletion in adult hippocampal neural stem/progenitor cells causes cellular abnormalities and alters neurogenesis. J Neurosci Society for Neuroscience 2012. April 25; 32(17):5880-90; http://dx.doi.org/ 10.1523/JNEUROSCI.5462-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Avitzour M, Mor-Shaked H, Yanovsky-Dagan S, Aharoni S, Altarescu G, Renbaum P, Eldar-Geva T, Schonberger O, Levy-Lahad E, Epsztejn-Litman S, et al.. FMR1 epigenetic silencing commonly occurs in undifferentiated Fragile X-affected embryonic stem cells. Stem Cell Reports 2014; 3:699-706; PMID:25418717; http://dx.doi.org/ 10.1016/j.stemcr.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Colak D, Zaninovic N, Cohen MS, Rosenwaks Z, Yang WY, Gerhardt J, Disney MD, Jaffrey SR. Promoter-bound trinucleotide repeat mRNA drives epigenetic silencing in Fragile X syndrome. Science 2014; 343:1002-5; PMID:24578575; http://dx.doi.org/ 10.1126/science.1245831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sheridan SD, Theriault KM, Reis SA, Zhou F, Madison JM, Daheron L, Loring JF, Haggarty SJ. Epigenetic characterization of the FMR1 gene and aberrant neurodevelopment in human induced pluripotent stem cell models of Fragile X syndrome. PLoS One 2011; 6:26203; http://dx.doi.org/ 10.1371/journal.pone.0026203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Urbach A, Bar-Nur O, Daley GQ, Benvenisty N. Differential modeling of Fragile X syndrome by human embryonic stem cells and induced pluripotent stem cells. Cell Stem Cell 2010; 6:407-11; PMID:20452313; http://dx.doi.org/ 10.1016/j.stem.2010.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]