Abstract
Background and objectives
Although prior studies have observed high resource use among patients with CKD, there is limited exploration of emergency department use in this population and the proportion of encounters related to CKD care specifically.
Design, setting, participants, & measurements
We identified all adults (≥18 years old) with eGFR<60 ml/min per 1.73 m2 (including dialysis-dependent patients) in Alberta, Canada between April 1, 2010 and March 31, 2011. Patients with CKD were linked to administrative data to capture clinical characteristics and frequency of emergency department encounters and followed until death or end of study (March 31, 2013). Within each CKD category, we calculated adjusted rates of overall emergency department use as well as rates of potentially preventable emergency department encounters (defined by four CKD-specific ambulatory care-sensitive conditions: heart failure, hyperkalemia, volume overload, and malignant hypertension).
Results
During mean follow-up of 2.4 years, 111,087 patients had 294,113 emergency department encounters; 64.2% of patients had category G3A CKD, and 1.6% were dialysis dependent. Adjusted rates of overall emergency department use were highest among patients with more advanced CKD; 5.8% of all emergency department encounters were for CKD-specific ambulatory care-sensitive conditions, with approximately one third resulting in hospital admission. Heart failure accounted for over 80% of all potentially preventable emergency department events among patients with categories G3A, G3B, and G4 CKD, whereas hyperkalemia accounted for almost one half (48%) of all ambulatory care-sensitive conditions among patients on dialysis. Adjusted rates of emergency department events for heart failure showed a U-shaped relationship, with the highest rates among patients with category G4 CKD. In contrast, there was a graded association between rates of emergency department use for hyperkalemia and CKD category.
Conclusions
Emergency department use is high among patients with CKD, although only a small proportion of these encounters is for potentially preventable CKD-related care. Strategies to reduce emergency department use among patients with CKD will, therefore, need to target conditions other than CKD-specific ambulatory care-sensitive conditions.
Keywords: chronic kidney disease; dialysis; Epidemiology and outcomes; Adult; Alberta; Ambulatory Care; Canada; Emergency Service, Hospital; Follow-Up Studies; heart failure; hospitalization; Humans; Hyperkalemia; Hypertension, Malignant; renal dialysis; Renal Insufficiency, Chronic
Introduction
CKD affects approximately 10% of the adult population and is associated with increased morbidity and mortality (1–5). Prior studies have also observed high resource use among patients with CKD (6–9). The majority of these studies have focused on either inpatient use or emergency department (ED) use among patients with ESRD (8,10–13). There has been limited exploration of how patients with CKD use the ED and whether utilization is associated with disease severity.
Although the ED is essential for providing urgent or emergent care, identifying ways of improving ED efficiency and decreasing wait times has been recognized as a priority in multiple countries (14–16). Improving coordination and management of care for patients with multiple chronic conditions (the norm for CKD) in an outpatient setting may meet health care needs and ultimately, improve patient experience and outcomes while reducing the burden currently placed on the ED (17–19). However, this requires an understanding of ED use among patients with CKD and the proportion of use that is amenable to outpatient care.
Using a large population-based cohort, we explored how rates of ED use vary by kidney disease severity among nondialysis- and dialysis-dependent patients with CKD and the proportion of these events that is potentially preventable by high-quality ambulatory care. This foundational information will help determine if specific subgroups of patients with CKD could be targeted to reduce ED use and improve patient experience and outcomes.
Materials and Methods
Data Sources and Study Population
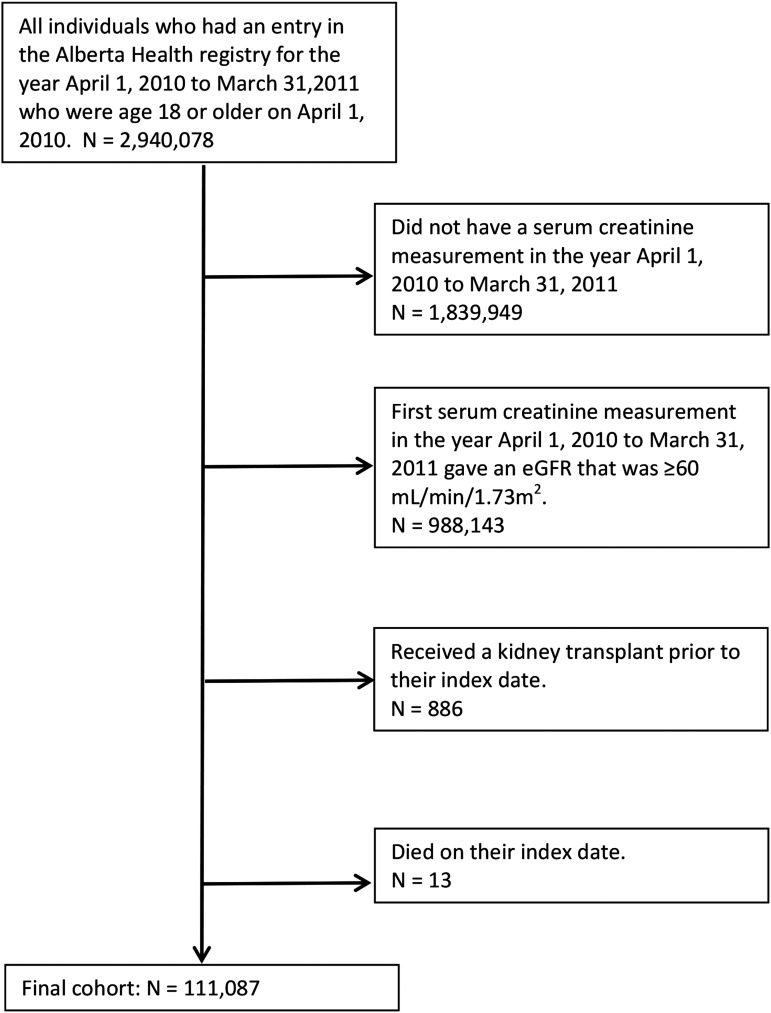
We conducted a retrospective cohort study using laboratory and administrative data from Alberta, Canada (20). The study population included Alberta residents ages 18 years old or older with CKD (nondialysis and dialysis dependent) between April 1, 2010 and March 31, 2011. CKD status was defined on the basis of the patient’s first (index) outpatient serum creatinine measurement in 2010–2011 that equated to eGFR of <60 ml/min per 1.73 m2 using the Chronic Kidney Disease Epidemiology Collaboration equation (21). CKD severity was categorized using Kidney Disease Improving Global Outcomes (KDIGO) guidelines as G3A (45–59 ml/min per 1.73 m2), G3B (30–44 ml/min per 1.73 m2), G4 (15–29 ml/min per 1.73 m2), and G5 (<15 ml/min per 1.73 m2) (22). Dialysis-dependent CKD (category G5D) was defined by receipt of chronic dialysis as captured by the provincial dialysis registry (23). We excluded patients who died on their index date and patients with kidney transplant before their index date due to inherent differences in health status and system usage within this patient subgroup (24,25).
Measurement of Covariates
Patients with CKD were linked to administrative data sources obtained from the provincial health ministry to capture additional clinical characteristics and measures of health care use. Patient-level characteristics included age, sex, registered First Nations status (all individuals registered under the Federal Indian Act), median neighborhood household income quintile, and urban/rural status. Presence of 28 comorbidities was ascertained on the basis of individual validated International Statistical Classification of Diseases and Health Related Problems, Ninth (ICD-9) and Tenth (ICD-10) Revisions coding algorithms (26). Presence of albuminuria was measured using a hierarchical combination of albumin-to-creatinine ratio (ACR), protein-to-creatinine ratio, or urine dipstick (UDIP; on the basis of availability) within the prior 2 years. Severe albuminuria (A3) was defined in accordance with KDIGO guidelines as ACR>300 mg/g (>30 mg/mmol), protein-to-creatinine ratio >500 mg/g (>50 mg/mmol), or UDIP of 2+ or more. Moderate albuminuria (A2) was defined as ACR between 30 and 300 mg/g (between 3 and 30 mg/mmol), protein-to-creatinine ratio from 150 to 500 mg/g (15–50 mg/mmol), or UDIP of trace or 1+. Normal/mild albuminuria (A1) was defined as ACR<30 mg/g (<3 mg/mmol), protein-to-creatinine ratio <150 mg/g (<15 mg/mmol), or normal UDIP (22). Outpatient primary care encounters in the 2-year period before index were categorized on the basis of the 25th and 75th percentiles as 0–7, 8–20, or >20 encounters. We also measured primary care continuity as defined by the proportion of outpatient primary care encounters made to a single provider in the prior 2 years (among those with at least three visits). This was categorized as 100%–75% (good attachment), 74%–50% (moderate attachment), or <50% (low attachment) (27,28). Finally, the proportion of patients with one or more visits to a nephrologist in the prior 2 years was captured.
Outcome—ED Utilization
We followed all patients from index date (first serum creatinine measurement that defined CKD status) until death, outmigration, or end of study (March 31, 2013). During this period, we recorded the count of ED encounters for each patient. We also identified specific encounter-level characteristics, including acuity (on the basis of the Canadian Emergency Department Triage and Acuity Scale [29] categorized as nonurgent, semiurgent, urgent, emergency, resuscitation, or unknown) and discharge disposition (admitted, died, or other). Multiple encounters on the same day were reported as one event. Of these multiple events, the one with the highest acuity or most serious discharge disposition was reported.
Identification of Potentially Preventable ED Use
Potentially preventable ED use was defined as acute care encounters for CKD-related ambulatory care-sensitive conditions (ACSCs). ACSCs are health conditions for which high-quality ambulatory care may prevent the need for acute care services (30,31), and they are recognized internationally as a quality indicator (32). Four CKD-specific ACSCs, including hyperkalemia, malignant hypertension, heart failure, and volume overload, were identified using previously defined ICD-9/ICD-10 coding algorithms (33,34). These were developed using a Delphi technique and have been used to identify ACSC encounters in patients with CKD in prior studies (34). The key difference between heart failure and volume overload as assessed using these algorithms is the latter comprising causes of extracellular fluid volume expansion not directly attributable to poor cardiac function.
Analyses
Patient characteristics and comorbidities were stratified by CKD category and described using proportions and means (SD) as appropriate. ED encounter-level characteristics were also reported across CKD category and reported as proportions. We also determined the top 10 most frequent diagnoses for all ED visits using the most responsible ICD-10 diagnostic code. Unadjusted rates of ED use (per 1000 person-years) were initially calculated. We then fit count models of the negative binomial family after testing for overdispersion, which was present in all patients, and estimated adjusted rates on the basis of these models. We adjusted for all of the demographic and clinical characteristics in Table 1, excluding nephrologist visits. Rates were adjusted to the sample proportions of the demographic and clinical characteristics of the cohort and reported by CKD category.
Table 1.
Cohort characteristics by CKD category (n=111,087)
| Characteristics | CKD Categorya | Overall, n=111,087 | ||||
|---|---|---|---|---|---|---|
| G3A, n=71,347 | G3B, n=28,020 | G4, n=8653 | G5 (Nondialysis), n=1305 | G5 (Dialysis), n=1762 | ||
| Demographic characteristics | ||||||
| Age, yr | ||||||
| 18–64 | 22.6 | 12.7 | 16.4 | 29.5 | 48.9 | 20.1 |
| 65–74 | 28.7 | 20.6 | 17.2 | 19.9 | 22.8 | 25.6 |
| 75–84 | 33.6 | 38.7 | 35.6 | 29.6 | 22.5 | 34.8 |
| ≥85 | 15.2 | 27.9 | 30.9 | 21.0 | 5.8 | 19.5 |
| Mean (SD) | 73.3 (11.8) | 77.8 (11.5) | 77.3 (13.1) | 72.1 (15.3) | 63.7 (15.8) | 74.6 (12.2) |
| Women | 58.0 | 59.6 | 57.7 | 51.2 | 41.8 | 58.1 |
| First Nations | 1.2 | 1.3 | 1.8 | 4.3 | 7.4 | 1.4 |
| Urban/rural | ||||||
| Urban | 77.5 | 76.8 | 77.8 | 76.2 | 81.1 | 77.4 |
| Rural | 22.4 | 23.0 | 22.1 | 23.6 | 18.8 | 22.5 |
| Unknown | 0.1 | 0.2 | 0.1 | 0.2 | 0.1 | 0.1 |
| Neighborhood income quintile (relative to Alberta) | ||||||
| 1 (Lowest) | 27.3 | 29.6 | 31.5 | 29.6 | 29.2 | 28.3 |
| 2 | 23.2 | 24.5 | 24.3 | 22.6 | 25.4 | 23.7 |
| 3 | 17.4 | 17.0 | 16.0 | 17.8 | 16.0 | 17.2 |
| 4 | 15.0 | 13.3 | 12.7 | 12.3 | 11.6 | 14.3 |
| 5 (Highest) | 14.6 | 12.7 | 12.2 | 13.2 | 12.9 | 13.9 |
| Unknown | 2.5 | 2.9 | 3.3 | 4.6 | 5.0 | 2.7 |
| Albuminuria | ||||||
| Normal/mild (A1) | 62.2 | 51.5 | 34.8 | 14.0 | 1.8 | 55.9 |
| Moderate (A2) | 16.5 | 21.8 | 25.9 | 21.1 | 5.4 | 18.5 |
| Severe (A3) | 3.8 | 8.7 | 21.9 | 44.1 | 39.0 | 7.4 |
| Unmeasured | 17.5 | 18.0 | 17.3 | 20.8 | 53.8 | 18.2 |
| Other comorbidities | ||||||
| Alcohol misuse | 2.7 | 3.2 | 4.2 | 6.1 | 7.6 | 3.1 |
| Asthma | 4.5 | 5.8 | 6.2 | 4.7 | 7.5 | 5.0 |
| Atrial fibrillation | 13.4 | 20.8 | 24.0 | 18.8 | 19.1 | 16.3 |
| Cancer—lymphoma | 0.8 | 1.0 | 1.4 | 1.6 | 2.0 | 1.0 |
| Cancer—metastatic | 1.6 | 2.2 | 2.2 | 2.9 | 2.9 | 1.8 |
| Cancer—nonmetastatic | 7.2 | 7.7 | 8.2 | 8.9 | 7.9 | 7.4 |
| Congestive heart failure | 14.4 | 27.0 | 38.2 | 34.6 | 43.9 | 20.2 |
| Chronic pain | 11.5 | 13.1 | 12.6 | 10.0 | 15.6 | 12.0 |
| Chronic pulmonary disease | 24.2 | 30.6 | 35.1 | 30.0 | 32.5 | 26.8 |
| Chronic viral hepatitis B | 0.1 | 0.1 | 0.1 | 0.2 | 0.8 | 0.1 |
| Cirrhosis | 0.4 | 0.7 | 1.0 | 0.8 | 1.9 | 0.6 |
| Dementia | 7.5 | 12.1 | 13.8 | 13.0 | 6.5 | 9.2 |
| Depression | 10.2 | 10.1 | 10.9 | 12.0 | 13.4 | 10.3 |
| Diabetes | 26.6 | 36.1 | 44.8 | 49.0 | 57.7 | 31.1 |
| Epilepsy | 1.8 | 1.8 | 2.4 | 2.6 | 5.3 | 1.9 |
| Hypertension | 77.6 | 90.2 | 93.2 | 90.4 | 93.7 | 82.4 |
| Hypothyroidism | 20.6 | 23.2 | 23.3 | 18.5 | 15.4 | 21.3 |
| Irritable bowel disease | 1.5 | 1.5 | 1.8 | 1.8 | 2.4 | 1.6 |
| Irritable bowel syndrome | 3.4 | 3.3 | 3.4 | 3.5 | 2.7 | 3.4 |
| Multiple sclerosis | 0.6 | 0.6 | 0.5 | 0.7 | 0.8 | 0.6 |
| Myocardial infarction | 7.8 | 12.0 | 15.2 | 15.0 | 17.8 | 9.7 |
| Parkinson disease | 0.9 | 1.3 | 1.3 | 1.4 | 1.6 | 1.0 |
| Peptic ulcer disease | 0.4 | 0.5 | 0.9 | 1.0 | 1.5 | 0.5 |
| Peripheral vascular disease | 3.6 | 5.9 | 7.8 | 7.0 | 27.9 | 4.9 |
| Psoriasis | 1.2 | 1.4 | 1.4 | 1.2 | 1.1 | 1.3 |
| Rheumatoid arthritis | 5.1 | 6.5 | 7.2 | 4.1 | 7.8 | 5.6 |
| Schizophrenia | 1.4 | 1.4 | 1.5 | 1.9 | 1.6 | 1.4 |
| Stroke | 16.9 | 23.5 | 27.0 | 24.7 | 25.3 | 19.6 |
| Total no. of chronic conditions, mean (SD) | 2.8 (2.0) | 3.8 (2.2) | 4.6 (2.3) | 4.5 (2.2) | 5.2 (2.4) | 3.3 (2.2) |
| Total no. of chronic conditions (excluding CKD), mean (SD) | 2.7 (1.9) | 3.4 (2.0) | 3.9 (2.1) | 3.7 (2.1) | 4.2 (2.4) | 3.0 (2.0) |
| Primary care use in 2 yr before index | ||||||
| No. of GP visits in 2 yr before index | ||||||
| 0–7 | 26.0 | 20.1 | 20.3 | 28.7 | 47.7 | 24.5 |
| 8–20 | 51.2 | 49.6 | 45.4 | 43.7 | 35.9 | 50.0 |
| >20 | 22.7 | 30.3 | 34.4 | 27.7 | 16.5 | 25.5 |
| Median (25th, 75th percentile) | 12 (7, 19) | 14 (9, 23) | 15 (9, 25) | 13 (6, 22) | 8 (3, 16) | 13 (8, 21) |
| GP continuity | ||||||
| Low (<50% with same GP) | 8.8 | 8.2 | 8.4 | 11.6 | 11.1 | 8.7 |
| Medium (50% to <75% with same GP) | 24.4 | 23.5 | 23.3 | 23.4 | 21.0 | 24.0 |
| High (≥75% with same GP) | 62.1 | 63.8 | 62.8 | 55.2 | 45.4 | 62.2 |
| Less than three GP visits (continuity not defined) | 4.7 | 4.5 | 5.5 | 9.8 | 22.5 | 5.1 |
| At least one visit with a nephrologist in prior 2 yr | 3.2 | 11.6 | 36.8 | 63.9 | 99.7 | 10.2 |
GP, general practitioner.
Percentage unless otherwise indicated.
We repeated our analysis for CKD-specific ACSC ED use. The total number of ACSC encounters, characteristics of the encounters, and relative proportion of each CKD-specific ACSC were reported by CKD category. Similarly, unadjusted and fully adjusted rates for any ACSC encounter were calculated. Adjusted rates for ED encounters related to heart failure and hyperkalemia were also calculated by CKD category. Similar analyses could not be completed for malignant hypertension and volume overload given the small number of events and unreliable estimates obtained from the regression models. We used STATA 11.2 for all analyses (Stata Corp., College Station, TX). The Conjoint Health Research Ethics Board of the University of Calgary approved this study and granted waiver of patient consent.
Results
We identified 1,100,129 adults with at least one outpatient serum creatinine during the study period. After excluding patients with eGFR≥60 ml/min per 1.73 m2 and those who received a prior kidney transplant or died on their index date, our final cohort included 111,087 patients (Figure 1). Within this cohort, the majority had category G3A CKD (64.2%), whereas 1.2% and 1.6% had nondialysis- and dialysis-dependent category G5 CKD, respectively. The mean (SD) age of the cohort was 74.6 (12.2) years old. Patients on dialysis were younger than those with categories G3, G4, and G5 (nondialysis) CKD. Comorbidity burden also increased with CKD category. The mean (SD) number of chronic conditions was 3.3 (2.2), whereas patients on dialysis had an average of 5.2 (2.4) conditions. Primary care use was high in the 2-year period before index, with a median of 13 (interquartile range, 8–21) visits. However, primary care continuity was lower among patients with more advanced CKD, with 11.1% of patients on dialysis having low continuity (<50% with the same primary care physician). Conversely, the proportion of patients with at least one nephrologist visit increased with severity of disease (Table 1).
Figure 1.
Flow diagram for cohort formation.
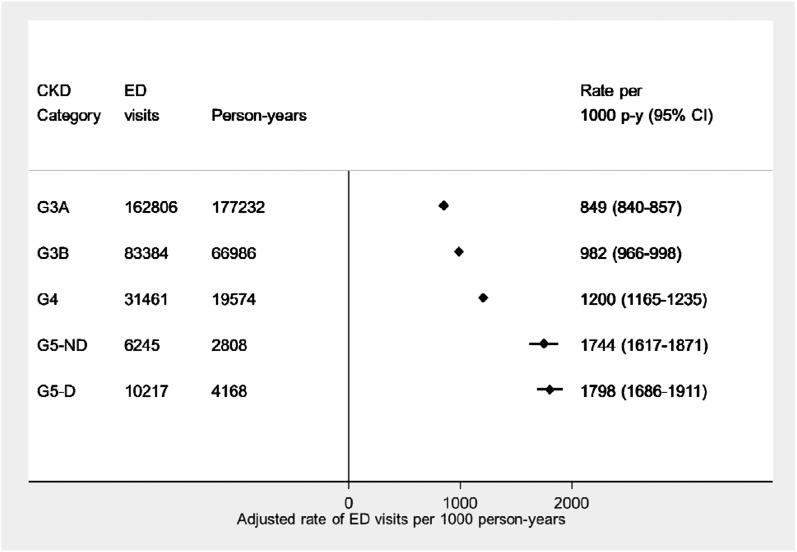
During an average follow-up of 2.4 years, 111,087 patients with CKD had 294,113 ED encounters (Table 2). Of these ED encounters, approximately one third resulted in an admission to the hospital, with similar proportions across disease severity. The acuity of the ED visits was often higher among patients with more advanced CKD, with patients on dialysis (category G5D) having a higher proportion of urgent or emergent visits to the ED. Figure 2 shows the graded association between adjusted rates of ED use and CKD category, with patients with G3A CKD having 849 (95% confidence interval [95% CI], 840 to 857) ED visits per 1000 person-years and patients on dialysis having 1798 (95% CI, 1686 to 1911) ED visits per 1000 person-years. The top 10 diagnoses for all ED visits among the CKD cohort are reported in Supplemental Appendix. The most frequent diagnoses were heterogeneous and included (but were not limited to) ICD-10 codes for antibiotic therapy, urinary tract infections, and pain-related complaints.
Table 2.
Encounter-level descriptive information for all emergency department visits in the CKD cohort (294,113 emergency department visits for 111,087 patients)
| Encounter-level Variables | CKD Category | ||||
|---|---|---|---|---|---|
| G3A, n=71,347 | G3B, n=28,020 | G4, n=8653 | G5 (Nondialysis), n=1305 | G5 (Dialysis), n=1762 | |
| No. of ED visits | 162,806 | 83,384 | 31,461 | 6245 | 10,217 |
| No. of person-yr | 177,232 | 66,986 | 19,574 | 2808 | 4168 |
| Unadjusted rate per 1000 person-yr (95% CI) | 919 (914 to 923) | 1245 (1236 to 1253) | 1607 (1590 to 1625) | 2224 (2169 to 2280) | 2451 (2404 to 2499) |
| ED visits by discharge disposition,a % | |||||
| Admitted | 23.9 | 29.8 | 35.1 | 33.0 | 33.0 |
| Died | 0.3 | 0.3 | 0.4 | 0.2 | 0.4 |
| Other | 75.8 | 69.8 | 64.4 | 66.8 | 66.5 |
| ED visits by acuity level,a % | |||||
| Nonurgent | 15.9 | 14.7 | 13.4 | 16.8 | 10.9 |
| Semiurgent | 28.4 | 27.4 | 25.6 | 24.0 | 22.1 |
| Urgent | 34.9 | 36.4 | 38.0 | 37.4 | 38.2 |
| Emergency | 13.7 | 14.7 | 16.4 | 15.8 | 22.4 |
| Resuscitation | 0.6 | 0.8 | 1.0 | 0.8 | 1.4 |
| Unknown | 6.4 | 6.0 | 5.6 | 5.2 | 5.0 |
ED, emergency department; 95% CI, 95% confidence interval.
Where there was more one ED visit in a day, the one that was counted was the one rated with the highest acuity or the one with the most serious discharge disposition.
Figure 2.
Plot of increasing adjusted rates (95% confidence interval [95% CI]) of all emergency department (ED) visits per 1000 person years (p-y) by CKD category. Rates were adjusted to the sample proportions of the demographic and clinical characteristics in the cohort. G5-D, dialysis dependent; G5-ND, nondialysis.
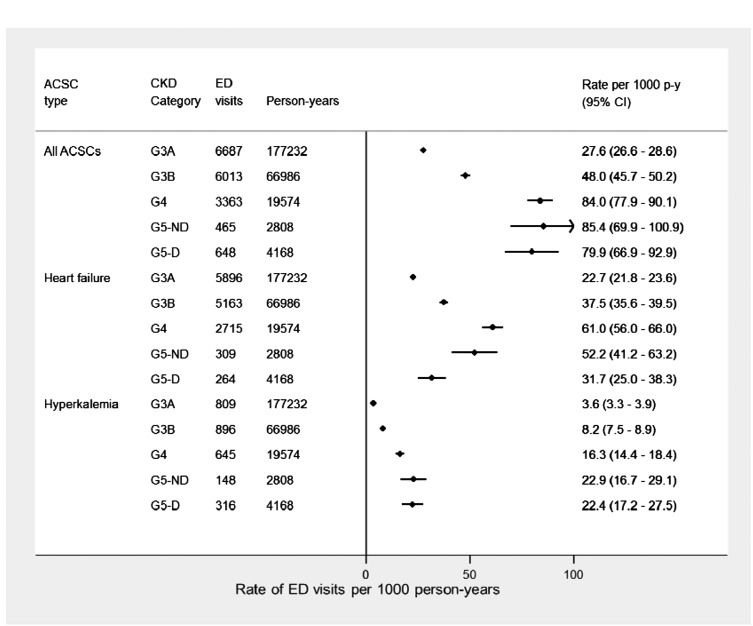
Of the total number of ED encounters, 17,176 (5.8%) were for CKD-specific ACSCs (Table 3). The majority of these ACSC encounters resulted in a hospital admission (70.5% for category G5 disease), whereas a higher proportion of encounters resulting in death was observed among patients on dialysis (1.1%). The acuity of these ED encounters also showed a higher proportion of resuscitations and emergency events for patients on dialysis. The most common ACSC was heart failure, accounting for over 80% of all potentially preventable events among patients in categories G3A, G3B, and G4 and 66% among patients in category G5 (nondialysis). Encounters for hyperkalemia were also common, particularly among patients on dialysis, in whom this condition accounted for almost one half (48.8%) of all ACSCs recorded. Volume overload was also substantially higher in patients on dialysis (16.8%) compared with those with lower categories of disease (ranging from 0.5% to 4.9%), whereas the proportion of ED encounters for malignant hypertension was similar across all categories of CKD.
Table 3.
Encounter-level descriptive information for emergency department visits for a CKD-related ambulatory care-sensitive condition (17,176 emergency department visits for 111,087 patients)
| Encounter-level Variables | CKD Category | ||||
|---|---|---|---|---|---|
| G3A, n=71,347 | G3B, n=28,020 | G4, n=8653 | G5 (nondialysis), n=1305 | G5 (dialysis), n=1762 | |
| No. of ED visits for a CKD-related ACSCa | 6687 | 6013 | 3363 | 465 | 648 |
| Person-time, person-yr | 177,232 | 66,986 | 19,574 | 2808 | 4168 |
| Unadjusted rate per 1000 person-yr (95% CI) | 38 (37 to 39) | 90 (88 to 92) | 172 (166 to 178) | 166 (151 to 181) | 155 (144 to 168) |
| ED visits for a CKD-related ACSC by discharge disposition,b % | |||||
| Admitted | 59.9 | 62.4 | 65.4 | 70.5 | 57.4 |
| Died | 0.5 | 0.4 | 0.4 | 0.2 | 1.1 |
| Other | 39.7 | 37.1 | 34.2 | 29.2 | 41.5 |
| ED visits for a CKD-related ACSC by acuity level,b % | |||||
| Nonurgent | 4.8 | 4.8 | 4.2 | 1.5 | 1.4 |
| Semiurgent | 15.8 | 15.1 | 14.7 | 15.1 | 7.3 |
| Urgent | 43.7 | 43.0 | 44.0 | 44.9 | 43.5 |
| Emergency | 30.3 | 30.8 | 32.3 | 32.5 | 42.3 |
| Resuscitation | 1.6 | 1.8 | 2.0 | 3.2 | 3.5 |
| Unknown | 3.7 | 4.5 | 2.8 | 2.8 | 2.0 |
| ED visits for a CKD-related ACSC by type of ACSC,a n (%) | |||||
| Heart failure | 5896 (88.2) | 5163 (85.9) | 2715 (80.7) | 309 (66.5) | 264 (40.7) |
| Hyperkalemia | 809 (12.1) | 896 (14.9) | 645 (19.2) | 148 (31.8) | 316 (48.8) |
| Volume overload | 39 (0.6) | 30 (0.5) | 62 (1.8) | 23 (4.9) | 109 (16.8) |
| Malignant hypertension | 24 (0.4) | 8 (0.1) | 8 (0.2) | 1 (0.2) | 2 (0.3) |
ED, emergency department; ACSC, ambulatory care-sensitive condition; 95% CI, 95% confidence interval.
The number represents the number of days with at least one CKD-related ACSC ED visit of that type, and therefore, the total number of CKD-related ACSC ED visits does not equal the total across type of ACSC.
Where there was more than one ED visit in a day, the one that was counted was the one rated with the highest acuity or the one with the most serious discharge disposition.
Adjusted rates for any ACSC encounters were higher among patients with more advanced CKD and highest among patients with category G5 CKD (nondialysis; 85.4; 95% CI, 69.9 to 100.9 ED visits per 1000 person-years) (Figure 3). When examining specific ACSCs, a U-shaped relationship was observed for encounters related to heart failure, with the highest ED rates among patients with category G4 (61.0; 95% CI, 56.0 to 66.0 ED visits per 1000 person-years) and lower rates among patients with category G5 CKD (nondialysis and dialysis dependent). A linear trend was observed for hyperkalemia, with the highest rates among patients with nondialysis-dependent category G5 CKD (22.9; 95% CI, 16.7 to 29.1 ED visits per 1000 person-years) and dialysis-dependent patients (22.4; 95% CI, 17.2 to 27.5 ED visits per 1000 person-years).
Figure 3.
Plot of adjusted rates (95% confidence intervals [95% CIs]) of emergency department (ED) visits for overall CKD-related ambulatory care sensitive conditions (ACSCs) per 1000 person years (p-y), heart failure, and hyperkalemia individually by CKD category. Rates were adjusted to the sample proportions of the demographic and clinical characteristics in the cohort. G5-D, dialysis dependent; G5-ND, nondialysis.
Discussion
In this large population-based cohort, we examined the association between CKD severity and ED utilization and found that overall rates of ED use were higher among patients with more advanced CKD and particularly high among dialysis-dependent patients. Furthermore, a small proportion (approximately 6%) of total ED use was for potentially preventable CKD-specific ACSCs. These findings suggest that strategies to reduce ED use among patients with CKD will need to be broad, although strategies targeting CKD-specific ACSCs may reduce a small but potentially important proportion of these ED encounters.
Prior studies have shown that health care use is high among patients with CKD and related to the medical complexity of this patient population (6–9,35). However, to our knowledge, few studies have examined the association between CKD category and use of ED services or have quantified the relative proportion of ED use that is for CKD-specific ACSCs. We found that the majority of CKD-specific ambulatory care-sensitive events were related to heart failure and hyperkalemia: conditions that are highly prevalent and key drivers of morbidity and mortality among patients with CKD (34,35). Despite the relatively low frequency of CKD-specific ACSCs, targeting patients with CKD at high risk of ED presentation for these two conditions could potentially improve the patient experience and clinical outcomes at modest cost if reductions in CKD-related ED use could be realized. However, any intervention would likely need to be modified for dialysis-dependent patients compared with those at milder stages of CKD.
With respect to prevention of hyperkalemia, educational strategies aimed at reducing sources of dietary potassium (36) and the use of standardized dialysate potassium protocols in the hemodialysis unit are examples of current strategies used for patients on dialysis (37,38). For heart failure, our results suggest that patients in earlier stages of disease progression could be targeted to potentially reduce the risk of ED encounters for this condition. Interventions to improve adherence to fluid restriction as well as methods of renin-angiotensin-aldosterone system inhibition have been shown to be effective therapies in heart failure, although the latter is not used in advanced CKD or among dialysis-dependent patients given the increased risk of hyperkalemia (39). Although this suggests that addressing hyperkalemia may improve the treatment of heart failure in this patient population, whether interventions aimed at one or both of these conditions could reduce ED utilization for hyperkalemia and/or heart failure is unknown and represents an area of future inquiry.
Regardless, our results must be put in context of overall ED use. The fact that 94% of ED encounters among patients with CKD were not related to CKD-specific care suggests that we should be focusing on not only interventions aimed at specific ACSCs per se but also, other medical conditions that seem to be driving overall ED use within this population. A better understanding of the comorbid profiles of patients with CKD, their health care needs, and the circumstances that result in presentation to the ED would be valuable when proposing strategies to improve care for patients with multimorbidity. This is particularly true for patients with early stages of CKD or underlying mental illness [a known driver of health care use in this population (34)], in whom early detection and integrated management strategies could mitigate downstream acute care use. Furthermore, improving coordination of care/attachment strategies for patients with CKD represents another avenue to potentially reduce overall ED use. We observed that primary care continuity decreased among patients with more advanced CKD; however, this may relate to the higher proportion of patients being cared for by a nephrologist with kidney disease progression. Greater continuity of care has been associated with decreases in ACSC encounters (40,41). Improving attachment to primary care among patients with CKD may, therefore, be an intervention that should be considered for future research in this patient population.
Our study should be interpreted in light of its limitations. First, we classified patients as having CKD on the basis of a single eGFR measurement, which may have resulted in misclassification. Second, the construct of ambulatory care-sensitive ED encounters likely represents a spectrum of preventability that requires consideration of other aspects of patient care, including use of outpatient and specialist services. Further to this point, CKD-specific ACSCs represent only a proportion of all ACSCs that have been proposed in the literature (42) and therefore, underestimate the total proportion of ED use that is potentially preventable. Third, two of four ACSCs considered were related to excess extracellular fluid volume. It is possible that there may be some overlap in the clinical application of the different administrative codes that constitute each condition, which may partially explain the U-shaped relationship observed between CKD category and rates of ED encounters for heart failure (i.e., a greater proportion of physicians coding heart failure as volume overload among dialysis-dependent patients). Fourth, although we did show that patients with more severe disease have lower primary care continuity, there are likely other important patient- and system-level factors related to coordination of care that may place patients with CKD at increased risk for ED use for CKD-specific events. Fifth, although we were able to measure a number of clinical and demographic characteristics, we were unable to account for all of the social determinants of health that may influence the risk of ED presentation. Despite these limitations, our study has as a number of strengths, including the use of population-based data within a single province of Canada, which provides a unique opportunity to assess the association between CKD and ED use. Furthermore, the use of linked provincial laboratory and administrative data sources also allowed for accurate identification of eGFR, albuminuria, and clinical characteristics as well as an in-depth exploration of ED use among this cohort.
Although the ED remains critical for providing care to those with acute medical needs, identifying ways of improving ED efficiency remains a priority. We found that ED use is high among patients with CKD, and a small but important proportion of these ED encounters is potentially preventable CKD-specific events. Future work is needed to determine if improvements in community-based care or dialysis treatments could potentially mitigate the number of CKD-related ED events for heart failure and hyperkalemia, respectively. However, strategies to target other chronic conditions (other than from CKD-specific conditions) will need to be considered in attempts to reduce overall ED use in this high-risk population. This will require a better understanding of the interplay between concurrent morbidity and health care needs among patients with CKD.
Disclosures
None.
Supplementary Material
Acknowledgments
M.T. is supported by the David Freeze Chair in Health Services Research. B.J.M. is supported by the Svare Professorship in Health Economics and a Health Scholar Award by Alberta Innovates Health Solutions (AIHS). P.R. is supported by operating grants from the Canadian Institutes of Health Research (CIHR). M.T.J. is supported by a New Investigator Award from the CIHR. B.R.H. is supported by the Roy and Vi Baay Chair in Kidney Research. The Interdisciplinary Chronic Disease Collaboration is funded by AIHS–Collaborative Research and Innovation Opportunity Team Grants Program.
This study is in part on the basis of data provided by Alberta Health and Alberta Health Services. The interpretation and conclusions are those of the researchers and do not represent the views of the Government of Alberta. Neither the Government of Alberta nor Alberta Health express any opinion in relation to this study. P.E.R. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors were responsible for revising the manuscript critically for important intellectual content, approved the final version, and agree to be accountable for all aspects of the work.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Preventing Emergency Department Use among Patients with CKD: It Starts with Awareness,” on pages 225–227.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06280616/-/DCSupplemental.
References
- 1.Drey N, Roderick P, Mullee M, Rogerson M: A population-based study of the incidence and outcomes of diagnosed chronic kidney disease. Am J Kidney Dis 42: 677–684, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Eknoyan G, Lameire N, Barsoum R, Eckardt KU, Levin A, Levin N, Locatelli F, MacLeod A, Vanholder R, Walker R, Wang H: The burden of kidney disease: Improving global outcomes. Kidney Int 66: 1310–1314, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, Quinn RR, Wiebe N, Tonelli M; Alberta Kidney Disease Network : Relation between kidney function, proteinuria, and adverse outcomes. JAMA 303: 423–429, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Zhang QL, Rothenbacher D: Prevalence of chronic kidney disease in population-based studies: Systematic review. BMC Public Health 8: 117, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumeister SE, Böger CA, Krämer BK, Döring A, Eheberg D, Fischer B, John J, Koenig W, Meisinger C: Effect of chronic kidney disease and comorbid conditions on health care costs: A 10-year observational study in a general population. Am J Nephrol 31: 222–229, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Honeycutt AA, Segel JE, Zhuo X, Hoerger TJ, Imai K, Williams D: Medical costs of CKD in the medicare population. J Am Soc Nephrol 24: 1478–1483, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinn RR, Ravani P, Zhang X, Garg AX, Blake PG, Austin PC, Zacharias JM, Johnson JF, Pandeya S, Verrelli M, Oliver MJ: Impact of modality choice on rates of hospitalization in patients eligible for both peritoneal dialysis and hemodialysis. Perit Dial Int 34: 41–48, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith DH, Gullion CM, Nichols G, Keith DS, Brown JB: Cost of medical care for chronic kidney disease and comorbidity among enrollees in a large HMO population. J Am Soc Nephrol 15: 1300–1306, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Chow E, Wong H, Hahn-Goldberg S, Chan CT, Morra D: Inpatient and emergent resource use of patients on dialysis at an academic medical center. Nephron Clin Pract 126: 124–127, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Hall RK, Toles M, Massing M, Jackson E, Peacock-Hinton S, O’Hare AM, Colón-Emeric C: Utilization of acute care among patients with ESRD discharged home from skilled nursing facilities. Clin J Am Soc Nephrol 10: 428–434, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harel Z, Wald R, McArthur E, Chertow GM, Harel S, Gruneir A, Fischer HD, Garg AX, Perl J, Nash DM, Silver S, Bell CM: Rehospitalizations and emergency department visits after hospital discharge in patients receiving maintenance hemodialysis. J Am Soc Nephrol 26: 3141–3150, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sacchetti A, Harris R, Patel K, Attewell R: Emergency department presentation of renal dialysis patients: Indications for EMS transport directly to dialysis centers. J Emerg Med 9: 141–144, 1991 [DOI] [PubMed] [Google Scholar]
- 14.Pines JM, Asplin BR, Kaji AH, Lowe RA, Magid DJ, Raven M, Weber EJ, Yealy DM: Frequent users of emergency department services: Gaps in knowledge and a proposed research agenda. Acad Emerg Med 18: e64–e69, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Roberge D, Pineault R, Larouche D, Poirier LR: The continuing saga of emergency room overcrowding: Are we aiming at the right target? Healthc Policy 5: 27–39, 2010 [PMC free article] [PubMed] [Google Scholar]
- 16.Canadian Institute for Health Information : Myth Busted! Emergency Room Overcrowding Is Caused by Non-Urgent Cases, Ottawa, ON, Canada, CIHI, 2009 [Google Scholar]
- 17.Hemmelgarn BR, Manns BJ, Zhang J, Tonelli M, Klarenbach S, Walsh M, Culleton BF: Association between multidisciplinary care and survival for elderly patients with chronic kidney disease. J Am Soc Nephrol 18: 993–999, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Hemmelgarn BR, Zhang J, Manns BJ, James MT, Quinn RR, Ravani P, Klarenbach SW, Culleton BF, Krause R, Thorlacius L, Jain AK, Tonelli M; Alberta Kidney Disease Network : Nephrology visits and health care resource use before and after reporting estimated glomerular filtration rate. JAMA 303: 1151–1158, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Ronksley PE, Hemmelgarn BR: Optimizing care for patients with CKD. Am J Kidney Dis 60: 133–138, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Hemmelgarn BR, Clement F, Manns BJ, Klarenbach S, James MT, Ravani P, Pannu N, Ahmed SB, MacRae J, Scott-Douglas N, Jindal K, Quinn R, Culleton BF, Wiebe N, Krause R, Thorlacius L, Tonelli M: Overview of the Alberta kidney disease network. BMC Nephrol 10: 30, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd , Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kidney Disease: Improving Global Outcomes : KDIGO 2012 clinical practice guidelines for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: 1–163, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Manns BJ, Mortis GP, Taub KJ, McLaughlin K, Donaldson C, Ghali WA: The Southern Alberta Renal Program database: A prototype for patient management and research initiatives. Clin Invest Med 24: 164–170, 2001 [PubMed] [Google Scholar]
- 24.Khan S, Tighiouart H, Kalra A, Raman G, Rohrer RJ, Pereira BJ: Resource utilization among kidney transplant recipients. Kidney Int 64: 657–664, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Schold JD, Elfadawy N, Buccini LD, Goldfarb DA, Flechner SM, P Phelan M, Poggio ED: Emergency department visits after kidney transplantation. Clin J Am Soc Nephrol 11: 674–683, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tonelli M, Wiebe N, Fortin M, Guthrie B, Hemmelgarn BR, James MT, Klarenbach SW, Lewanczuk R, Manns BJ, Ronksley P, Sargious P, Straus S, Quan H; Alberta Kidney Disease Network : Methods for identifying 30 chronic conditions: Application to administrative data. BMC Med Inform Decis Mak 15: 31, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaakkimainen RL, Klein-Geltink J, Guttmann A, Barnsley J, Jagorski B, Kopp A: Indicators of Primary Care Based on Administrative Data, Toronto, Institute for Clinical Evaluative Sciences, 2006 [Google Scholar]
- 28.Jee SH, Cabana MD: Indices for continuity of care: A systematic review of the literature. Med Care Res Rev 63: 158–188, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Bullard MJ, Unger B, Spence J, Grafstein E; CTAS National Working Group : Revisions to the Canadian Emergency Department Triage and Acuity Scale (CTAS) adult guidelines. CJEM 10: 136–151, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Billings J, Anderson GM, Newman LS: Recent findings on preventable hospitalizations. Health Aff (Millwood) 15: 239–249, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Billings J, Zeitel L, Lukomnik J, Carey TS, Blank AE, Newman L: Impact of socioeconomic status on hospital use in New York City. Health Aff (Millwood) 12: 162–173, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Sanmartin C, Khanand S; The LHAD research team : Hospitalizations for Ambulatory Care Sensitive Conditions (ACSC): The Factors That Matter, Ottawa, ON, Canada, Statistics Canada, 2011 [Google Scholar]
- 33.Gao S, Manns BJ, Culleton BF, Tonelli M, Quan H, Crowshoe L, Ghali WA, Svenson LW, Ahmed S, Hemmelgarn BR; Alberta Kidney Disease Network : Access to health care among status Aboriginal people with chronic kidney disease. CMAJ 179: 1007–1012, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiebe N, Klarenbach SW, Allan GM, Manns BJ, Pelletier R, James MT, Bello A, Hemmelgarn BR, Tonelli M; Alberta Kidney Disease Network : Potentially preventable hospitalization as a complication of CKD: A cohort study. Am J Kidney Dis 64: 230–238, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Tonelli M, Wiebe N, Guthrie B, James MT, Quan H, Fortin M, Klarenbach SW, Sargious P, Straus S, Lewanczuk R, Ronksley PE, Manns BJ, Hemmelgarn BR: Comorbidity as a driver of adverse outcomes in people with chronic kidney disease. Kidney Int 88: 859–866, 2015 [DOI] [PubMed] [Google Scholar]
- 36.Garagarza CA, Valente AT, Oliveira TS, Caetano CG: Effect of personalized nutritional counseling in maintenance hemodialysis patients. Hemodial Int 19: 412–418, 2015 [DOI] [PubMed] [Google Scholar]
- 37.Kovesdy CP: Management of hyperkalaemia in chronic kidney disease. Nat Rev Nephrol 10: 653–662, 2014 [DOI] [PubMed] [Google Scholar]
- 38.Perazella MA: Approach to hyperkalemic end-stage renal disease patients in the emergency department. Conn Med 63: 131–136, 1999 [PubMed] [Google Scholar]
- 39.Epstein M: Hyperkalemia constitutes a constraint for implementing renin-angiotensin-aldosterone inhibition: The widening gap between mandating treatment guidelines and the real-world clinical arena. Kidney Int Suppl 6: 20–28, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Canadian Institute for Health Information : Continuity of Care with Family Medicine Physicians: Why It Matters, Ottawa, ON, Canada, CIHI, 2015 [Google Scholar]
- 41.Menec VH, Sirski M, Attawar D, Katz A: Does continuity of care with a family physician reduce hospitalizations among older adults? J Health Serv Res Policy 11: 196–201, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Canadian Institute for Health Information : Technical Note: Ambulatory Care Sensitive Conditions (ASCS), Ottawa, ON, Canada, CIHI, 2010 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.