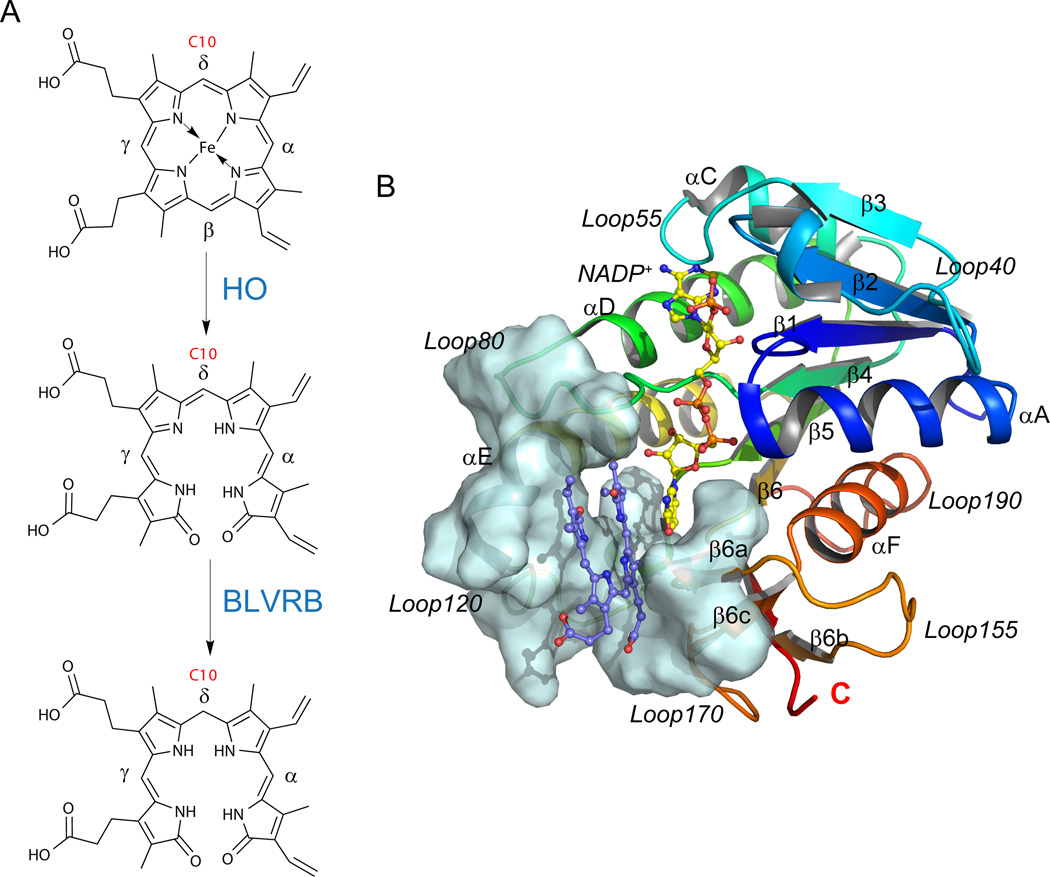
Fig. 1.
BLVRB structure and its role in heme catabolism. (A) Reaction scheme of the heme degradation pathway, focusing on the step catalyzed by BLVRB. BLVRB displays a preference for biliverdin isomers without propionates straddling the C10 position (red); HO represents heme oxygenase. (B) Overall structure of the BLVRB ternary complex (PDB entry 1HE2; [12] BLVRB with NADP+ and biliverdin IXα). The active site around the substrate is shown in pale cyan surface, including a two-stranded parallel β-sheet (β6a and β6c), the N-terminus of αE helix, the flexible Loop80 and Loop120.