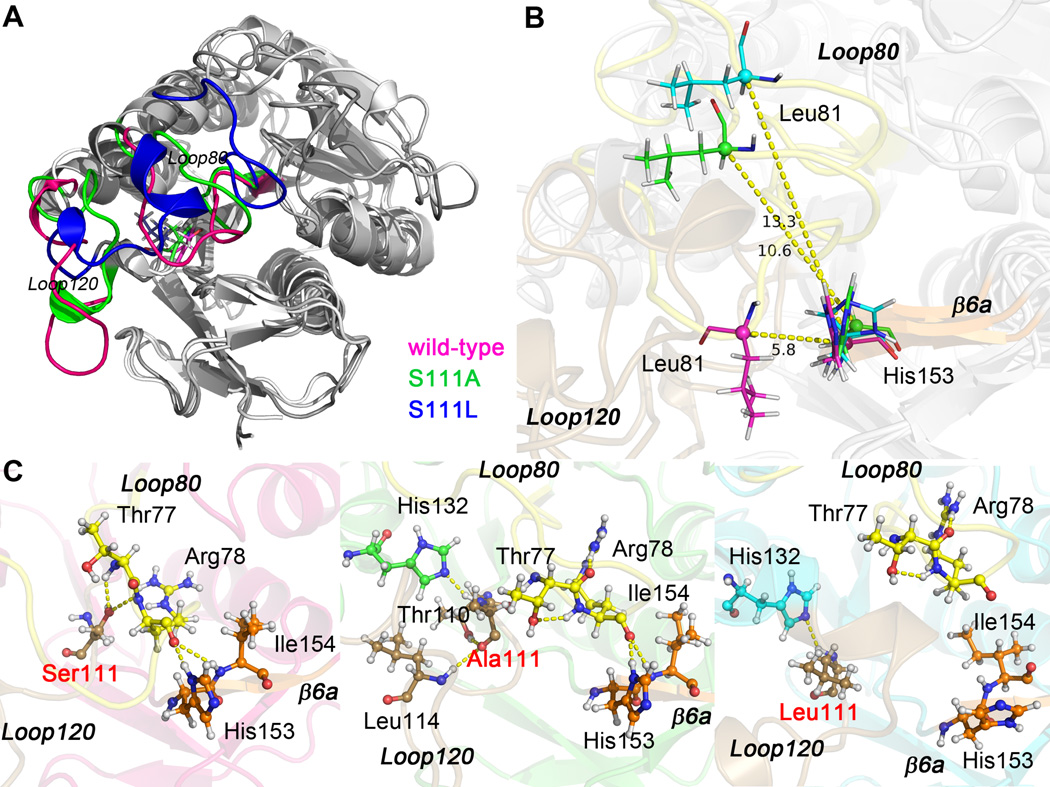
Fig. 4.
Superposed structures of frame 100 ns of apo wild-type, apo Ser111Ala, and apo Ser111Leu BLVRB. (A) Loop80 and Loop120 are colored for different systems (wild-type, pink; Ser111Ala, green; Ser111Leu, blue), residue 111 is shown in sticks. (B) Distance between Cα atom of Leu81 and Cα atom of His153. Loop80, Loop120, and β6a are labeled and colored in yellow, brown, and orange, respectively. (C) Hydrogen bonding network within Loop80 (yellow), Loop120 (brown), and β6a (orange) of apo wild-type, apo Ser111Ala, and apo Ser111Leu BLVRB. The mutation site is labeled in red, and hydrogen bonds are illustrated by yellow dashed lines.