Abstract
Poor social networks and decreased levels of social support are associated with worse mood, health, and cognition in younger and older adults. Yet, we know very little about the brain substrates associated with social networks and social support, particularly in older adults. This study examined functional brain substrates associated with social networks using the Social Network Index (SNI) and resting-state functional magnetic resonance imaging (fMRI). Resting-state fMRI data from 28 non-demented older adults were analyzed with independent components analyses. As expected, four established resting-state networks—previously linked to motor, vision, speech, and other language functions—correlated with the quality (SNI-1: total number of high-contact roles of a respondent) and quantity (SNI-2: total number of individuals in a respondent’s social network) of social networks: a sensorimotor, a visual, a vestibular/insular, and a left frontoparietal network. Moreover, SNI-1 was associated with greater functional connectivity in the lateral prefrontal regions of the left frontoparietal network, while SNI-2 was associated with greater functional connectivity in the medial prefrontal regions of this network. Thus, lateral prefrontal regions may be particularly linked to the quality of social networks while medial prefrontal regions may be particularly linked to the quantity of social networks.
Keywords: Social Network Index, resting-state fMRI, older adults
Introduction
Social support and social networks are distinct, but interrelated psychological constructs. Social support is the perception that an individual is cared for and has support available if needed, the actual assistance that is received, and or the level of integration an individual has in a social network (Antonucci, 1990). A social network refers to a social structure consisting of social interactions and relationships (e.g., family, friends, coworkers, and neighbors) that an individual can turn to for support.
The Social Network Index (SNI) is a widely used measure of social networks (Cohen, Doyle, Skoner, Rabin, & Gwalyney, 1997). The SNI measures an individual’s high-contact roles (SNI-1: the number of social roles that the respondent has contact with at least biweekly) and the number of people in an individual’s social network (SNI-2: total number of people in the respondents’ social network). The SNI-1 and SNI-2 scores can be thought of as reflecting the quality and the quantity of social interactions and relationships, respectively, as SNI-1 score is the number of individuals one has biweekly contact with and SNI-2 is the overall number of individuals, regardless of frequency of communication.
The benefits of robust social support and social networks in older adults are well supported in the literature. Increased social networks are associated with enhanced mental and physical health (Cohen & Syme, 1985) and high levels of morale in older adults (Loke, Abdullah, Chai, Hamid, & Yahaya, 2011). More importantly, for the present purposes, greater social support is associated with better cognitive functions in cognitively healthy older adults (Pillemer & Holtzer, 2015), and robust social networks and higher levels of perceived social support may have a protective effect against cognitive decline (Barnes, De Leon, Wilson, Bienias, & Evans, 2004; Bassuk, Glass, & Berkman, 1999; Ertel, Glymour, & Berkman, 2008; Fratiglioni, Paillard-Borg, & Winblad, 2004; James, Wilson, Barnes, & Bennett, 2011; Zunzunegui, Alvarado, Del Ser, & Otero, 2003) and dementia (Fabrigoule, Letenneur, Dartigues, & Zarrouk, 1995). Older adults that are more socially integrated (as assessed by marital status, volunteer activity, and how often the respondent has contact with children, parents, and neighbors), for example, exhibit slower age-related memory decline than older adults that are less socially integrated (Ertel et al., 2008).
Despite a well-established link between social support and well-being in older adults, the structural and functional brain substrates associated with social support and social networks are not well known. A fairly large body of evidence, however, suggests that there are age-related changes to the structure and function of the brain (Craik & Rose, 2012; Daselaar et al., 2001; Morcom, Good, Frackowiak, & Rugg, 2003; Raz, 2000; Raz et al., 1997; Raz & Rodrigue, 2006; Stebbins et al., 2002), and that these changes are different in individuals with mild cognitive impairment (MCI) and Alzheimer’s disease (AD; Celone et al., 2006; Driscoll et al., 2009; Kalpouzos et al., 2009; Whitwell et al., 2007). Given the differences discussed above, it is critical to first establish brain correlates of social support in normal aging as a prelude to understanding how these brain structures/functional networks change due to neurodegenerative disease.
Structural magnetic resonance imaging (MRI) studies have identified positive associations between social network size and gray matter volume in the ventrome-dial prefrontal cortex (Lewis, Rezaie, Brown, Roberts, & Dunbar, 2011) and the amygdala (Bickart, Wright, Dautoff, Dickerson, & Barrett, 2011), in young and middle-aged adults, respectively. Yet, at this time the structural brain correlates of social support and social networks in older adults are unknown. In addition, there are no task-related functional MRI (fMRI) studies examining the functional brain correlates of social support or social networks in younger or older adults—presumably because of the challenges associated with designing an fMRI task that assesses social support or social networks.
Task-related fMRI studies of self-referential processing (Gutchess, Kensinger, & Schacter, 2007; Kelley et al., 2002) and socially mediated memory distortions (Edelson, Sharot, Dolan, & Dudai, 2011), however, provide a window into the functional brain systems that are involved in social processing in general. Gutchess and colleagues (2007), for example, found that self-referencing (as assessed by adjectives related to self versus another person) was associated with neural activation in medial prefrontal and midcingulate cortices in both younger and older adults. Self-referencing in older adults also engaged supplementary motor, primary somatosensory, and cerebellar regions to a greater extent than self-referencing in younger adults. The role of the medial prefrontal cortex during social processing has been further specified by showing that neural activation in the ventromedial prefrontal cortex is associated with processing information related to self, neural activation in the dorsomedial prefrontal cortex is associated with processing information related to strangers, and, finally, the dorsomedial and ventromedial prefrontal cortex is associated with processing information related to close others (Johnson et al., 2002; Kelley et al., 2002; Krienen, Tu, & Buckner, 2010; Moran, Lee, & Gabrieli, 2010; Zhu, Zhang, Fan, & Han, 2007). Moreover, Edelson and colleagues (2011) found that socially mediated memory distortions (as assessed by exposing individuals to erroneous recollections generated by others) in younger adults were associated with neural activation in amygdala and hippocampal regions. Taken together, these task-related fMRI studies suggest that medial prefrontal, cingulate, amygdala, and hippocampal regions are engaged during social processing in general.
Resting-state fMRI (rfMRI) studies examine functional connectivity while the brain is at rest (Biswal, Kylen, & Hyde, 1997; Biswal, Zerrin Yetkin, Haughton, & Hyde, 1995) and are considered to reflect, and are generally consistent with, underlying anatomical connections between brain regions. Such studies have consistently identified resting-state networks that, in turn, are associated with different cognitive and motor functions (Damoiseaux et al., 2006; Smith et al., 2009; Van Den Heuvel & Pol, 2010). The two visual resting-state networks, for example, are generally linked to visual functions, the sensorimotor network to bimanual motor functions, the vestibular/insular network to speech functions, the two frontoparietal networks to language (left-lateralized fronto-parietal network) and somesthesis (right-lateralized fronto-parietal network), and the executive control network to executive functions. The default mode network is a resting-state network that is consistently deactivated during a wide variety of externally motivated cognitive tasks and is therefore considered to be involved in internally motivated cognition such as reminiscence or imagining the future (Buckner, Andrews-Hanna, & Schacter, 2008; Raichle et al., 2001). The default mode network is the most studied resting-state network in older adults and has been shown to be disrupted during normal aging as well as in individuals with MCI and AD (Andrews-Hanna et al., 2007; Damoiseaux et al., 2008; Grecius, Srivastava, Reiss, & Menon, 2004; Hafkemeijer, van der Grond, & Rombouts, 2012). Given the challenges associated with designing an fMRI task that assesses social support or social networks, rfMRI is particularly suited for examining the functional brain systems associated with social support and social networks in older adults.
There is a paucity of rfMRI studies investigating the relationship between social networks and resting-state networks in older adults. One rfMRI study has linked amygdala–cortical connectivity to social networks size in younger adults (Bickart, Hollenbeck, Barrett, & Dickerson, 2012), but resting-state connectivity associated with social networks has not been examined in older adults. As mentioned previously, identifying resting-state networks associated with social networks is important because there are known age-related and AD-related changes to the structure and function of the brain (Craik & Rose, 2012; Daselaar et al., 2001; Morcom et al., 2003; Raz, 2000; Raz et al., 1997; Raz & Rodrigue, 2006; Stebbins et al., 2002). Identifying the neural systems associated with social networks may also shed light on the ways in which social support influences health, mortality, and well-being, as well as inform the development of interventions that can be specifically targeted or optimized for different older adult populations. Determining whether the brain systems associated with social networks are particularly spared or particularly affected by normal aging, MCI, or AD, for example, could be useful for determining who will benefit from interventions focused on building, maintaining, or strengthening social support and social networks.
Our specific aim for this study was to identify functional resting-state networks associated with the quality and quantity of social networks in non-demented, community-dwelling older adults. Using the SNI (Cohen et al., 1997), we examined the shared and distinct relationship between functional resting-state networks and the quality and quantity of social networks. Given that social support has been linked to social, physical, as well as cognitive well-being in the past, we hypothesized that several resting-state networks, rather than one specific resting-state network, would be associated with the quality and quantity of social networks in older adults—and therefore used a whole-brain, multivariate analytic approach to explore this issue.
Methods
Participants
The sample (N = 28) consisted of community-residing non-demented older adults enrolled in a longitudinal cohort study entitled “Central Control of Mobility in Aging.” The primary aim of the study is to examine cognitive predictors of mobility function, mobility decline, and disability in the aging population (Holtzer, Wang, & Verghese, 2014; Holtzer et al., 2014). Participants received a telephone interview prior to inclusion in the study, which consisted of verbal consent to participate in the study, a medical history questionnaire, a mobility questionnaire, and cognitive screens to rule out dementia (Buschke et al., 1999; Galvin et al., 2005). Additionally, the participants were required to be residents of Westchester County, New York, at least 65 years of age, and English-speaking. General exclusion criteria consisted of severe auditory or visual disturbances that would interfere with testing, self-reported cognitive difficulties, inability to walk the length of a room or climb stairs without assistive devices or assistance from another person, any medical/neurological history that may interfere with performance on cognitive and motor tests, and a diagnosis of dementia, as diagnosed via consensus diagnostic case conference procedures once the participant is enrolled (Holtzer, Verghese, Wang, Hall, & Lipton, 2008). MRI-specific exclusion criteria included left-handedness, which was assessed using the Edinburgh Handedness Inventory (Oldfield, 1971), claustrophobia, and surgically implanted metallic devices (e.g., pacemaker).
After completing the phone interview and meeting eligibility, participants were invited for two 3-h visits at the research center. The Albert Einstein College of Medicine Institutional Review Board approved the study and written informed consent is obtained from all subjects prior to participation. Participants completed two visits for the calendar year (about a week apart). On the first visit, participants completed a battery of neuropsychological tests, a walking paradigm, and a series of questionnaires. On the second visit, participants received a full neurological examination, walking and balance measures, computerized cognitive paradigms, and a series of questionnaires. Participants in the Central Control of Mobility in Aging study are followed longitudinally at yearly intervals. If a participant is interested and eligible for MRI, he or she is evaluated to determine eligibility. If participants are deemed eligible for MRI, they are invited back for a third visit in which they will undergo imaging. During rfMRI acquisition, each participant was instructed to relax, clear their minds, and try not to think of anything in particular when they are undergoing the brain scan. Prior to imaging, the participants have each completed the SNI on the second study visit of the year.
A total of 28 participants who completed the SNI questionnaire and underwent rfMRI with less than 2 mm of movement in any direction (x, y, or z) were included in this study. Baseline demographic characteristics are presented in Table 1. The study sample (N = 28) had a mean age of 72.71(± 5.25) years, a mean education level of 15.07 (± 2.73) years, and a gender distribution of 57% female. The mean Repeatable Battery for Assessment of Neuropsychological Status (RBANS) standardized total score (95.46, ±11.60) was in the average range of cognitive function. The low disease comorbidity summary score (1.29, ±1.08) was indicative of relatively good health.
Table 1.
Descriptive statistics of demographic information (N = 28).
| M (SD) | Range | |
|---|---|---|
| Age (years) | 72.71 (5.25) | 65–87 |
| Gender (% female) | 57.14 | |
| Education (years) | 15.07 (2.73) | 12–20 |
| Global health status score | 1.29 (1.08) | 0–3 |
| RBANS (standard total score) | 95.46 (11.60) | 78–119 |
| RBANS Immediate Memory Index | 100.07 (10.99) | 81–122 |
| RBANS Delayed Memory Index | 95.54 (11.15) | 76–118 |
| RBANS Attention Index | 104.04 (14.13) | 62–134 |
| RBANS Visuospatial Index | 92.79 (12.53) | 62–118 |
| RBANS Language Index | 95.68 (10.16) | 65–115 |
| SNI-1 Score | 5.54 (1.64) | 1–9 |
| SNI-2 Score | 28.25 (27.07) | 4–138 |
Global health status score (range 0–10) obtained from dichotomous rating (presence or absence) of diabetes, chronic heart failure, arthritis, hypertension, depression, stroke, Parkinson’s disease, chronic obstructive pulmonary disease, angina, and myocardial infarction; RBANS: Repeatable Battery for the Assessment of Neuropsychological Status; SNI-1: Social Network Index 1 Score; SNI-2: Social Network Index 2 Score.
Measures
Social Network Index
The SNI is a tool used to assess involvement in 12 different types of social relationships (Cohen et al., 1997). The SNI examines relationships with a spouse, parents, parents-in-law, children, close family members and friends, coworkers, classmates, volunteers, religious groups, and other groups. The SNI is divided into two scores: SNI-1 and SNI-2. The SNI-1 score represents the number of high-contact roles of a particular individual. The respondent is in touch with high-contact roles at least biweekly. The maximum number of high-contact roles an individual can have is 12, and these include: spouse, parent, child, child-in-law, close relative, close friend, religious group member, student, employee, neighbor, volunteer, and group member. The SNI-2 score represents the total number of people with whom the respondent has contact. This score is computed by summing up the number of people the respondent has contact with across the 12 domains (Cohen et al., 1997).
Global cognitive functioning was assessed using the RBANS total score. Although considered a screening battery (see measures for details), the variability of the RBANS total scores among non-demented older adults is substantial as evidenced by published normative data (Duff et al., 2008). The RBANS total score is a standardized score with a mean of 100 and a standard deviation of 15 (Duff et al., 2008). Sub-scores for immediate memory, delayed memory, attention, visuospatial, and language abilities can also be obtained. A disease comorbidity summary score (ranging from 0 to 10) was computed by the presence or absence of diabetes, chronic heart failure, arthritis, hypertension, depression, stroke, Parkinson’s disease, chronic obstructive lung disease, angina, and myocardial infarction, as previously described (Holtzer et al., 2008).
MRI data acquisition
MRI scanning was performed using a Philips 3T Achieva Quasar TX multinuclear MRI/MRS (Magnetic Resonance Spectroscopy) system equipped with a Dual Quasar High Performance Gradient System, 32-channel broadband digital RF system, Quadrature T/R Head Coil, RapidView reconstructor, Intera Achieva ScanTools Pro R2.5 Package, NetForum and ExamCards, and SENSE parallel imaging capability. The T1-weighted whole head structural image was acquired using axial 3D-MP-RAGE (magnetic prepared rapid acquisition gradient echo) parameters over a 240 mm field of view (FOV) and 1.0 mm isotropic resolution, TE = 4.6 ms, TR = 9.9 ms, a = 8o, with SENSE factor 2.5. Blood-oxygen-level dependent (BOLD; T2*-weighted) images were obtained with echo planar imaging using a whole-brain gradient over a 240 mm FOV on a 128 × 128 acquisition matrix, 3 mm slice thickness (no gap); TE = 30 ms, TR = 2000 ms, flip angle = 90° and 42 trans-axial slices per volume. A neuroradiologist reviewed each MRI scan to verify that there were no clinically significant findings for any of the participants. During the rfMRI task, the participants were asked to keep their eyes closed, lie still in the scanner, and not fall asleep for the 6 min of recording time (Van Dijk et al., 2010).
MRI image preprocessing
fMRI image preprocessing was performed using Functional MRI of the Brain (FMRIB) Software Library (FSL) (Version 4.1), FSL (http://fsl.fmrib.ox.ac.uk/fsl) (Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012; Smith et al., 2004; Woolrich et al., 2009) and consisted of non-brain removal using brain extraction tool (Smith, 2002), motion correction with motion correction using FMRIB’s linear image registration tool (Jenkinson et al., 2012; Jenkinson and Smith, 2001), slice-timing correction for interleaved acquisitions using Fourier-space time-series phase shifting, high-pass temporal filtering using Gaussian-weighted least-squares straight line fitting (σ = 50 s); spatial smoothing using a Gaussian kernel with full-width half-maximum 8 mm, co-registration to high-resolution T1-weighted images, and normalization to standard space (Montreal Neurological Institute atlas, using resolutions of 4 × 4 × 4 mm) using combined affine and nonlinear registration (FSL FMRIB’s nonlinear image registration tool, with warp resolution = 10 mm).
Statistical analysis
Independent components analysis
For each participant, the normalized fMRI images were concatenated across time to form a single 4D image. This 4D image was then analyzed with FSL Multivariate Exploratory Linear Optimized Decomposition into Independent Components (MELODIC 3.0 software, Version 4.1; Beckmann & Smith, 2004). MELODIC is an independent component analysis (ICA) that separates the BOLD signal into statistically independent spatial components and their associated time series. This ICA permits the removal of structured and random noise, and the isolation of task-activated neural networks (Beckmann, DeLuca, Devlin, & Smith, 2005; Cole, Smith, & Beckmann, 2010; Fox & Raichle, 2007; Greicius et al., 2004; Murphy, Birn, & Bandettini, 2013), and has been frequently used to identify resting-state low-frequency neural networks in the past (Beckmann et al., 2005; Beckman & Smith, 2004; Cole et al., 2010; Fox & Raichle, 2007; Greicius et al., 2004; Murphy et al., 2013). We used this technique to identify functional resting-state networks that correlated with SNI-1 and SNI-2 scores in two separate regression models. Based on previous rfMRI studies, we limited our ICA analyses to 20 components that correlated with these two distinct aspects of social networks (Smith et al., 2009). Criterion for statistical significance was set at p < .05.
Manual classification of components
Even after conventional preprocessing steps, several confounding factors including head movement and physiological activity could compromise interpretation of rfMRI data (Bhaganagarapu, Jackson, & Abbott, 2013; Kelly et al., 2010; Power, Barnes, Snyder, Schlaggar, & Petersen, 2012). In fact, the removal of artifacts related to participant motion and physiological sources (e.g., cardiac and respiratory cycles) is imperative to limit illegitimate findings of rfMRI analyses (Murphy et al., 2013; Thomas, Harshman, & Menon, 2002). To classify independent components generated in our ICA analyses as artifacts or neural signals of interest, we employed an operationalized fMRI denoising procedure that has been shown to be reliable and to enhance the sensitivity of results from rfMRI data (Kelly et al., 2010). According to this denoising procedure, components are considered artifactual if >90% of activation or deactivation is observed in peripheral regions, or in a random scattered pattern over one-fourth or more of the brain without correspondence to functional-anatomical boundaries. By contrast, components are considered neural signals of interest if >10% of activation or deactivation is observed in small to large gray matter clusters in non-peripheral regions. Additional criteria for noise considered with this denoising procedure are high-frequency activity, spikes, sinus coactivation, and saw tooth pattern.
Results
Of the 20 components generated from our ICA that correlated with SNI-1 and SNI-2 scores, 16 components were determined to be artifactual following the operationalized fMRI denoising procedure. The remaining four components were determined to be neural signals of interest (see Figure 1). All anatomical and functional descriptions were classified with reference to the underlying standard-space images in concordance with several atlases (Lancaster et al., 1997, 2000). The components were then compared to previously identified, well-established resting-state networks derived from large meta-analyses (Smith et al., 2009; Ystad et al., 2011).
Figure 1.
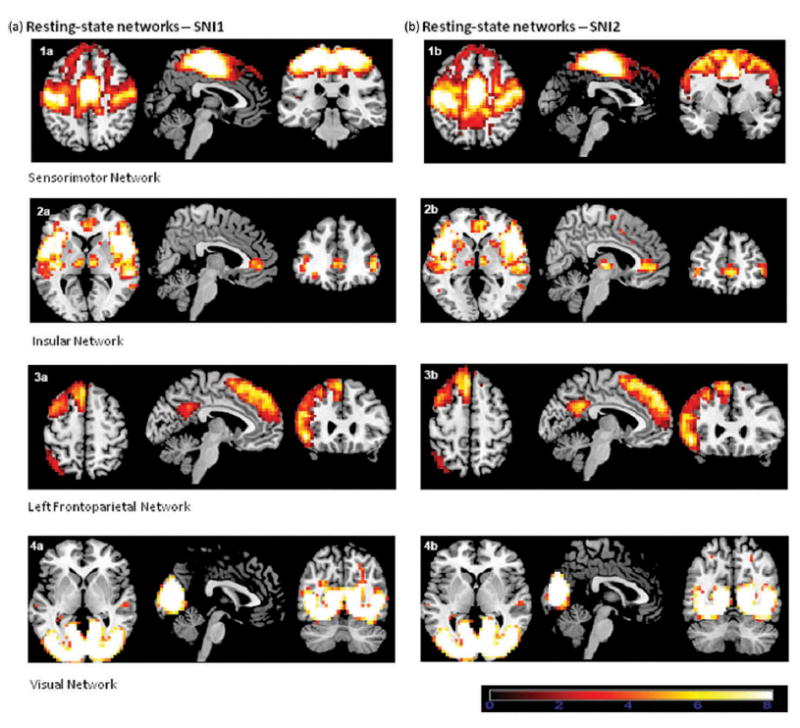
Resting-state networks associated with SNI 1 score (a) and SNI2 score (b). This figure shows the three most informative axial, sagittal, and coronal slices of each resting-state network superimposed on the Montreal Neurologic Institute (MNI) template supplied by MRIcron software. The left side of the image corresponds to the left side of the brain. All ICA spatial maps were converted to z statistic images via a normalized mixture-model fit, and then thresholded at z = 2.30.
As expected, SNI-1 and SNI-2 scores were correlated, r = .58, p = .001, df = 26. The SNI-1 and SNI-2 was not associated with global cognition as assessed with RBANS total scores, r = .181, p = .357, df = 26 and r = .110, p = .577, df = 26, respectively. Using linear regression analysis among the five cognitive domains or tests that contribute to the RBANS total score, only the delayed memory score was associated with SNI-1, B = .061, R = .412, p = .029. More importantly, for the present purposes, SNI-1 and SNI-2 scores were associated with four resting-state networks: a sensorimotor, a vestibular/insular, a visual, and a left frontoparietal network. The first shared network (sen-sorimotor) was bilateral and included the primary motor cortex, the somatosensory cortex, and the dorsomedial prefrontal cortex. The sensorimotor network is often bilateral and has been consistently identified during rest (Beckmann et al., 2005; Biswal et al., 1997; Smith et al., 2009). The second shared network (visual) was also bilateral and encompassed the primary and secondary visual cortices. Associations between this network and functionally identified visual regions have been well established in the rfMRI literature (Beckmann & Smith, 2005). The third network (vestibular/insular) was also bilateral and included the insula and the ventromedial prefrontal cortex. The fourth shared network (left frontoparietal) was left-lateralized, and included the left dorsomedial prefrontal cortex, left ventrolateral prefrontal cortex, left posterior cingulate, and the precuneus. Frontoparietal networks have been strongly lateralized in the rfMRI literature (Smith et al., 2009).
SNI-1 score compared with SNI-2 score
In general, we observed comparable associations between SNI-1 and SNI-2, and sensorimotor, visual, and vestibular/insular networks. Notable differences, however, were visually observed in the left frontoparietal network (see Figure 2). While SNI-1 was associated with greater functional connectivity in the lateral pre-frontal cortex regions of the left frontoparietal network, SNI-2 was associated with greater functional connectivity in the medial prefrontal cortex regions of this network. Our analytic approach did not permit us to formally evaluate these differences, and therefore these findings should be replicated and/or evaluated with other analytic methods in upcoming studies.
Figure 2.
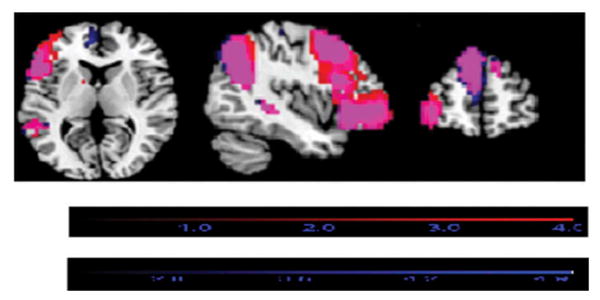
Overlay of the left frontoparietal resting-state networks associated with SNI1 (red) and SNI2 (blue) on the same template. The purple color represents the areas in which the networks overlap. The red color shows where the SNI1 networks extend beyond the SNI2 networks. The blue color shows where the SNI2 networks extend beyond the SNI1 networks.
Discussion
The present study revealed that four resting-state networks were associated with the quality and quantity of social networks in non-demented older adults: a sensorimotor, a visual, a vestibular/insular, and a left frontoparietal network. We also found that the quality of social networks in older adults was associated with greater connectivity in the lateral prefrontal portions of the left frontoparietal network, while the quantity of social networks in older adults was associated with greater connectivity in the medial prefrontal regions of this network. The resting-state networks associated with social networks in the present study encompass brain regions that have been linked to social processing in task-related fMRI studies and highlight that these regions do not work in isolation, but are components of stable resting-state neural networks that are important for a wide range of perceptual, cognitive, and motor functions.
Increases in the quality and quantity of social networks were associated with greater functional connectivity in a sensorimotor resting-state network. This network was composed of brain regions in the primary motor cortex, the somatosensory cortex, and the dorsomedial prefrontal cortex. This sensorimotor resting-state network has been previously linked to bimanual motor actions (Biswal et al., 1995; Smith et al., 2009), including complex motor actions such as walking and walking while talking (Yuan, Blumen, Verghese, & Holtzer, 2015). Shared functional brain substrates between social networks and motor actions are expected because individuals with robust social networks are more physically active than individuals with poor social networks (for a review, see McNeill, Kreuter, & Subramanian, 2006). Shared functional brain substrates between social networks and motor actions are also expected because late-life participation in social activities has been shown to reduce age-related motor decline (Buchman et al., 2009). The dorsomedial pre-frontal regions of this resting-state network contained supplementary motor regions previously linked to motor planning as well as dorsomedial prefrontal regions previously linked to processing social information related to strangers and close others (Goldberg, 1985; Johnson et al., 2002; Kelley et al., 2002; Krienen et al., 2010; Moran et al., 2010; Nachev, Kennard, & Husain, 2008; Zhu et al., 2007).
Improved quality and quantity of social networks in older adults were also associated with greater functional connectivity in a bilateral visual resting-state network. This resting-state network was primarily composed of the primary and secondary visual cortices, and as mentioned previously, associations between this network and functionally identified visual processing regions are well established (Beckmann & Smith, 2005). Visual perception is also important for processing socially relevant information and can influence, and be influenced by, social cognition and social behaviors (Adolphs, 2001). Shared functional brain substrates of social networks and visual processing are also expected because participation in social activities has been shown to reduce age-related decline in visual processing speed (Lövden et al., 2005).
Enhanced quality and quantity of social networks in older adults were also associated with greater functional connectivity in a vestibular/insular resting-state network. More specifically, greater connectivity in the insula and ventromedial prefrontal cortex were associated with greater quality and quantity of social networks in our sample of non-demented older adults. This vestibular/insular network has been consistently linked to the perception and execution of speech (Beckmann & Smith, 2005), which is an integral part of social communication. Task-based fMRI studies have also linked the ventromedial prefrontal cortex to self-referencing, person perception, self-reflection, and emotional support (Gutchess et al., 2007; Johnson et al., 2002; Kelley et al., 2002; Krienen et al., 2010; Onoda et al., 2009), and the insula to emotion, self-awareness, and interpersonal experiences (Murray, Debbané, Fox, Bzdok, & Eickhoff, 2014).
The fourth shared resting-state network associated with the quality and quantity of social networks in older adults was a left-lateralized frontoparietal network, which included lateral and dorsomedial prefrontal regions, the posterior cingulate, and the precuneus. Recent literature has focused on the lateralization and distinct functions of frontoparietal resting-state networks (Smith et al., 2009). In general, the left-lateralized frontoparietal network has been linked to language functions, and the right-lateralized frontoparietal network has been linked to somesthesis. More specifically, the left-lateralized frontoparietal network has been linked to language and facial recognition (Leube, Erb, Grodd, Bartels, & Kircher, 2003), both of which are key components of social communication and interaction. Task-based fMRI studies have also linked the cingulate to perceived partner support (Eisenberger, Taylor, Gable, Hilmert, & Lieberman, 2007) and the precuneus to self-attribution (Cabanis et al., 2013).
While the quality of social networks in older adults was associated with greater connectivity in the lateral pre-frontal regions of the left frontoparietal network, the quantity of social networks in older adults was associated with greater connectivity in the medial prefrontal regions of this network. This finding is interesting in given the old age of our participants because medial prefrontal regions are less susceptible to age-related atrophy than lateral prefrontal regions (Salat et al., 2004; Sowell et al., 2003). As mentioned previously, the SNI-1 score encompasses the number of high-contact social roles in which the participant reports regular contact (at least once every 2 weeks), and the SNI-2 score encompasses the total number of people with whom the participant has contact. Thus, this finding suggests that maintaining high-quality social relationships may be more dependent on lateral prefrontal regions, and consequently more sensitive to age-related changes (Lieberman, 2007). However, future studies that contrasts the resting-state networks associated with the quality and quantity of social networks in young and old, or examine the resting-state networks associated with the quality and quantity of social networks in a longitudinal sample of older adults, are needed to confirm this proposition.
Limitations
Several limitations of this study should be considered. The participants in this study were all community-dwelling, cognitively healthy older adults. Therefore, the results of the current study may not be generalizable to older adults with significant physical and/or cognitive impairments, such as older adults with Parkinson’s disease, MCI, or AD. Future studies are needed to extend and/or delineate the boundaries of these findings in larger, more diverse populations of older adults. Without a young or a middle-aged comparison group (or longitudinal data), it is also unclear if the relationships between social networks and resting-state networks observed in this study are age-general or age-specific. Without a formal evaluation of the differences in functional connectivity associated with SNI-1 and SNI-2, these differences also need to be replicated and/or evaluated with different analytic methods in upcoming studies. Additionally, as the SNI is a self-report measure, there are some concerns that participants may exaggerate or underreport their perceived level of social support to either make their situation seem worse or minimize their problems, respectively.
Conclusions and future research recommendations
As expected, the present study showed that the quality and quantity of social networks in older adults were associated with four well-established sensorimotor, vestibular/insular, visual, and left frontoparietal resting-state networks. These resting-state networks have been previously linked to a wide range of motor, visual, speech, and (other) language functions. As such, the link between these functional brain systems and social networks in older adults is consistent with studies showing that robust social support and social networks are associated with a wide range of positive outcomes, including mental, physical, and cognitive well-being. In addition, the present study provided preliminary support for a differential relationship between resting-state networks and the number of high-contact roles (SNI-1) and the quantity of individuals (SNI-2) in a social network. More specifically, higher levels of high-contact roles were associated with greater connectivity in the lateral prefrontal regions of the left frontoparietal network, while a higher number of individuals in a social network were associated with greater connectivity in the medial prefrontal regions of this network. This finding suggests that different brain substrates may be important for maintaining the number of high-contact roles and the overall number of members in a social network. To our knowledge, this is the first study to use rfMRI to examine the functional brain systems associated with social networks in older adults.
In order to directly examine the resting-state networks associated with social networks as a function of normal and pathological aging, future research should contrast the resting-state networks associated with social network in non-demented older adults with younger and/or middle-aged adult cohorts, as well as older adults with MCI and AD. The relationship between social networks, resting-state networks, and different cognitive domains also needs further study. We have previously shown that perceived social support is associated with global cognition (Pillemer & Holtzer, 2015). In the current study of a fairly small sample of non-demented older adults, the quality and quantity of social networks were not associated with global cognition, yet the quality of social networks was associated with delayed memory performance. Future studies of larger, and more cognitively diverse samples of older adults, will determine if these effects are indeed reliable. Such investigative efforts will increase knowledge of the brain systems associated with social networks and might also lead to targeted interventions designed to promote social engagement in older adults.
Acknowledgments
This research was supported by 1RO1AG036920-01A1: NIH/NIA, Holtzer, R. (PI). Helena M. Blumen was also supported by 5KL2TR001071-03 (NIH) and the Resnick Gerontology Center at Albert Einstein College of Medicine.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Adolphs R. The neurobiology of social cognition. Current Opinion in Neurobiology. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56(5):924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonucci TC. Social supports and social relationships. Handbook of Aging and the Social Sciences. 1990;3:205–226. [Google Scholar]
- Barnes LL, De Leon CM, Wilson RS, Bienias JL, Evans DA. Social resources and cognitive decline in a population of older African Americans and whites. Neurology. 2004;63(12):2322–2326. doi: 10.1212/01.WNL.0000147473.04043.B3. [DOI] [PubMed] [Google Scholar]
- Bassuk SS, Glass TA, Berkman LF. Social disengagement and incident cognitive decline in community-dwelling elderly persons. Annals of Internal Medicine. 1999;131(3):165–173. doi: 10.7326/0003-4819-131-3-199908030-00002. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society B: Biological Sciences. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Transactions Medica Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Tensorial extensions of independent component analysis for multisubject FMRI analysis. Neuroimage. 2005;25(1):294–311. doi: 10.1016/j.neuroimage.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Bhaganagarapu K, Jackson GD, Abbott DF. An automated method for identifying artifact in independent component analysis of resting-state fMRI. Frontiers Human Neuroscience. 2013;7(343):10–3389. doi: 10.3389/fnhum.2013.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickart KC, Hollenbeck MC, Barrett LF, Dickerson BC. Intrinsic amygdala –cortical functional connectivity predicts social network size in humans. The Journal of Neuroscience. 2012;32(42):14729–14741. doi: 10.1523/JNEUROSCI.1599-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickart KC, Wright CI, Dautoff RJ, Dickerson BC, Barrett LF. Amygdala volume and social network size in humans. Nature Neuroscience. 2011;14(2):163. doi: 10.1038/nn.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995;34(4):537–541. doi: 10.1002/(ISSN)1522-2594. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Kylen JV, Hyde JS. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR in Biomedicine. 1997;10(4–5):165–170. doi: 10.1002/(SICI)1099-1492(199706/08)10:4/5<165∷AID-NBM454>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Buchman AS, Boyle PA, Wilson RS, Fleischman DA, Leurgans S, Bennett DA. Association between late-life social activity and motor decline in older adults. Archives of Internal Medicine. 2009;169(12):1139–1146. doi: 10.1001/archinternmed.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124(1):1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buschke H, Kuslansky G, Katz M, Stewart WF, Sliwinski MJ, Eckholdt HM, Lipton RB. Screening for dementia with the memory impairment screen. Neurology. 1999;52(2):231–231. doi: 10.1212/WNL.52.2.231. [DOI] [PubMed] [Google Scholar]
- Cabanis M, Pyka M, Mehl S, Müller BW, Loos-Jankowiak S, Winterer G, Kircher T. The precuneus and the insula in self-attributional processes. Cognitive, Affective, & Behavioral Neuroscience. 2013;13(2):330–345. doi: 10.3758/s13415-012-0143-5. [DOI] [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL. Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: An independent component analysis. The Journal of Neuroscience. 2006;26(40):10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwalyney JM., Jr Social ties and susceptibility to the common cold. Journal of the American Medical Association. 1997;277:1940–1944. doi: 10.1001/jama.1997.03540480040036. [DOI] [PubMed] [Google Scholar]
- Cohen SE, Syme S. Social support and health. New York, NY: Academic Press; 1985. [Google Scholar]
- Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Frontiers Systems Neuroscience. 2010;4:8. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FI, Rose NS. Memory encoding and aging: A neurocognitive perspective. Neuroscience & Biobehavioral Reviews. 2012;36(7):1729–1739. doi: 10.1016/j.neubiorev.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita ES, Barkhof F, Scheltens P, Stam CJ, Rombouts SARB. Reduced resting-state brain activity in the “default network” in normal aging. Cerebral Cortex. 2008;18(8):1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Rombouts SA, Veltman DJ, Raaijmakers JG, Lazeron RH, Jonker C. Parahippocampal activation during successful recognition of words: A self-paced event-related fMRI study. NeuroImage. 2001;13:1113–1120. doi: 10.1006/nimg.2001.0758. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Davatzikos C, An Y, Wu X, Shen D, Kraut M. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology. 2009;72(22):1906–1913. doi: 10.1212/WNL.0b013e3181a82634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K, Humphreys Clark JD, O’Bryant SE, Mold JW, Schiffer RB, Sutker PB. Utility of the RBANS in detecting cognitive impairment associated with Alzheimer’s disease: Sensitivity, specificity, and positive and negative predictive powers. Archives of Clinical Neuropsychology: the Official Journal of the National Academy of Neuropsychologists. 2008;23(5):603–612. doi: 10.1016/j.acn.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelson M, Sharot T, Dolan RJ, Dudai Y. Following the crowd: Brain substrates of long-term memory conformity. Science. 2011;333(6038):108–111. doi: 10.1126/science.1203557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD. Neural pathways link social support to attenuated neuroendocrine stress responses. Neuroimage. 2007;35(4):1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertel KA, Glymour MM, Berkman LF. Effects of social integration on preserving memory function in a nationally representative US elderly population. Journal Information. 2008;98(7):1215–1220. doi: 10.2105/AJPH.2007.113654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrigoule C, Letenneur L, Dartigues JF, Zarrouk M. Social and leisure activities and risk of dementia: A prospective longitudinal study. Journal of the American Geriatrics Society. 1995 doi: 10.1111/j1532-5415.1995.tb06093.x. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. The Lancet Neurology. 2004;3(6):343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Roe CM, Powlishta KK, Coats MA, Muich SJ, Grant E, Morris JC. The AD8 A brief informant interview to detect dementia. Neurology. 2005;65(4):559–564. doi: 10.1212/01.wnl.0000172958.95282.2a. [DOI] [PubMed] [Google Scholar]
- Goldberg G. Supplementary motor area structure and function: Review and hypotheses. Behavioral and Brain Sciences. 1985 Apr 8;8:567–588. doi: 10.1017/S0140525X00045167. [DOI] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proceedings of the National Academy of Sciences. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Engelien W, Feng H, Silbersweig DA, Stern E, Yang Y. Mapping transient, randomly occurring neuropsychological events using independent component analysis. Neuroimage. 2001;14(6):1432–1443. doi: 10.1006/nimg.2001.0914. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Schacter DL. Aging, self-referencing, and medial prefrontal cortex. Social Neuroscience. 2007;2:117–133. doi: 10.1080/17470910701399029. [DOI] [PubMed] [Google Scholar]
- Hafkemeijer A, van der Grond J, Rombouts SA. Imaging the default mode network in aging and dementia. Biochimica Et Biophysica Acta (Bba)-Molecular Basis of Disease. 2012;1822(3):431–441. doi: 10.1016/j.bbadis.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Holtzer R, Verghese J, Wang C, Hall CB, Lipton RB. Within-person across-neuropsychological test variability and incident dementia. JAMA. 2008;300(7):823–830. doi: 10.1001/jama.300.7.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Wang C, Verghese J. Performance variance on walking while talking tasks: Theory, findings, and clinical implications. Age. 2014;36(1):373–381. doi: 10.1007/s11357-013-9570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James BD, Wilson RS, Barnes LL, Bennett DA. Late-life social activity and cognitive decline in old age. Journal of the International Neuropsychological Society. 2011 Jun 17;:998–1005. doi: 10.1017/S1355617711000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125(8):1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Kalpouzos G, Chételat G, Baron J-C, Landeau B, Mevel K, Godeau C. Voxel-based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiology of Aging. 2009;30(1):112–124. doi: 10.1016/j.neurobiolaging.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14(5):785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kelly RE, Jr, Alexopoulos GS, Wang Z, Gunning FM, Murphy CF, Morimoto SS, Hoptman MJ, et al. Visual inspection of independent components: Defining a procedure for artifact removal from fMRI data. Journal of Neuroscience Methods. 2010;189:233–245. doi: 10.1016/j.jneumeth.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen FM, Tu PC, Buckner RL. Clan mentality: Evidence that the medial prefrontal cortex responds to close others. Journal of Neuroscience. 2010;30(41):13906–13915. doi: 10.1523/JNEUROSCI.2180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, Mazziotta JC, et al. Automated labeling of the human brain: A preliminary report on the development and evaluation of a forward-transform method. Human Brain Mapping. 1997;5:238–242. doi: 10.1002/(SICI)1097-0193(1997)5:41.0.CO;2-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Fox PT, et al. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/(ISSN)1097-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leube DT, Erb M, Grodd W, Bartels M, Kircher TT. Successful episodic memory retrieval of newly learned faces activates a left fronto-parietal network. Cognitive Brain Research. 2003;18(1):97–101. doi: 10.1016/j.cogbrainres.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Rezaie R, Brown R, Roberts N, Dunbar RI. Ventromedial prefrontal volume predicts understanding of others and social network size. Neuroimage. 2011;57(4):1624–1629. doi: 10.1016/j.neuroimage.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: A review of core processes. Annual Reviews Psychologist. 2007;58:259–289. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Loke SC, Abdullah SS, Chai ST, Hamid TA, Yahaya N. Assessment of factors influencing morale in the elderly. Plos One. 2011;6(1) doi: 10.1371/journal.pone.0016490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lövdén M, Ghisletta P, Lindenberger U. Social participation attenuates decline in perceptual speed in old and very old age. Psychology and Aging. 2005;20(3):423–434. doi: 10.1037/0882-7974.20.3.423. [DOI] [PubMed] [Google Scholar]
- McNeill LH, Kreuter MW, Subramanian SV. Social environment and physical activity: A review of concepts and evidence. Social Science & Medicine. 2006;63(4):1011–1022. doi: 10.1016/j.socscimed.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Moran JM, Lee SM, Gabrieli JDE. Dissociable neural systems supporting knowledge about human character and appearance in ourselves and others. Journal of Cognitive Neuroscience. 2010;23(9):2222–2230. doi: 10.1162/jocn.2010.21580. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Good CD, Frackowiak RS, Rugg MD. Age effects on the neural correlates of successful memory encoding. Brain. 2003;126(1):213–229. doi: 10.1093/brain/awg020. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Bandettini PA. Resting-state fMRI confounds and cleanup. Neuroimage. 2013;80:349–359. doi: 10.1016/j.neuroimage.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RJ, Debbané M, Fox PT, Bzdok D, Eickhoff SB. Functional connectivity mapping of regions associated with self and other processing. Human Brain Mapping. 2014;36(4):1304–1324. doi: 10.1002/hbm.22703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nature Reviews Neuroscience. 2008 Nov 9;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Nakashima KI, Nittono H, Ura M, Yamawaki S. Decreased ventral anterior cingulate cortex activity is associated with reduced social pain during emotional support. Social Neuroscience. 2009;4(5):443–454. doi: 10.1080/17470910902955884. [DOI] [PubMed] [Google Scholar]
- Pillemer SC, Holtzer R. The differential relationships of dimensions of perceived social support with cognitive function among older adults. Aging & Mental Health. 2015:1–9. doi: 10.1080/13607863.2015.1033683. ahead-of-print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In: Raz N, Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 2. Mahwah (NJ): Lawrence Erlbaum Associates Publishers; 2000. pp. 1–90. [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD. Selective aging of the human cerebral cortex observed in vivo: Differential vulnerability of the prefrontal gray matter. Cerebral Cortex. 1997;7(3):268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neuroscience & Biobehavioral Reviews. 2006;30(6):730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E. Thinning of the cerebral cortex in aging. Cerebral Cortex. 2004;14(7):721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Matthews PM, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nature Neuroscience. 2003;6(3):309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Stebbins GT, Carrillo MC, Dorfman J, Dirksen C, Desmond JE, Turner DA, Gabrieli JD, et al. Aging effects on memory encoding in the frontal lobes. Psychology and Aging. 2002;17(1):44–55. doi: 10.1037/0882-7974.17.1.44. [DOI] [PubMed] [Google Scholar]
- Thomas CG, Harshman RA, Menon RS. Noise reduction in BOLD-based fMRI using component analysis. Neuroimage. 2002;17:1521–1537. doi: 10.1006/nimg.2002.1200. [DOI] [PubMed] [Google Scholar]
- Van Den Heuvel MP, Pol HEH. Exploring the brain network: A review on resting-state fMRI functional connectivity. European Neuropsychopharmacology. 2010;20(8):519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: Theory, properties, and optimization. Journal of Neurophysiology. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Przybelski SA, Weigand SD, Knopman DS, Boeve BF, Petersen RC, Jack CR. 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer’s disease. Brain. 2007;130(7):1777–1786. doi: 10.1093/brain/awm112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Smith SM, et al. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45:S173–S86. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Ystad M, Hodneland E, Adolfsdottir S, Haasz J, Lundervold AJ, Eichele T, Lundervold A. Cortico-striatal connectivity and cognition in normal aging: A combined DTI and resting state fMRI study. Neuroimage. 2011;55:24–31. doi: 10.1016/j.neuroimage.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Yuan J, Blumen HM, Verghese J, Holtzer R. Functional connectivity associated with gait velocity during walking and walking-while-talking in aging: A resting-state fMRI study. Human Brain Mapping. 2015;36:1484–1493. doi: 10.1002/hbm.22717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Zhang L, Fan J, Han S. Neural basis of cultural influence on self-representation. Neuroimage. 2007;34(3):1310–1316. doi: 10.1016/j.neuroimage.2006.08.047. [DOI] [PubMed] [Google Scholar]
- Zunzunegui MV, Alvarado BE, Del Ser T, Otero A. Social networks, social integration, and social engagement determine cognitive decline in community-dwelling Spanish older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2003;58(2):93–100. doi: 10.1093/geronb/58.2.S93. [DOI] [PMC free article] [PubMed] [Google Scholar]


