Abstract
Subjective responses to alcohol are considered candidate endophenotypes for alcohol use disorder (AUD) and appear to anticipate future consumption. However, prospective studies have been rare, and laboratory research has typically examined subjective responses absent measures of self-administration. This study examined the association of subjective responses with subsequent laboratory self-administration, also evaluating laboratory phenotypes in relation to putative genetic risk factors (family history [FH] of alcohol dependence and OPRM1 genotype) and subsequent heavy drinking. Participants (N=61, M=19.89 years, SD=0.86) completed laboratory sessions involving intravenous alcohol challenge (Session 1) and free-access intravenous self-administration (Session 2), followed by prospective assessments. Multilevel modeling showed that higher reported stimulation and lower sedation during Session 1 independently predicted greater alcohol self-administration during Session 2. Although self-administration did not differ by FH group, participants with the OPRM1 118G allele evidenced steeper BrAC trajectories and greater peak BrAC relative to 118A homozygous participants. Prospective analyses supported significant indirect associations between Session 1 subjective responses and 6-month heavy drinking via peak BrAC in Session 2. Additionally, significant indirect associations of FH (via Session 1 stimulation and Session 2 peak BrAC) and OPRM1 (via peak BrAC) with follow-up heavy drinking were observed. These results further support the utility of human laboratory phenotypes in prospective studies of AUD risk, and highlight the potential role of self-administration phenotypes in longitudinal research.
Keywords: Alcohol administration, alcohol clamp, Asn40Asp, development, opioid receptor, rs1799971
Introduction
Individual differences in acute responses to alcohol, commonly indexed by subjective reports during laboratory alcohol challenge, have emerged as one of few promising candidate endophenotypes for alcohol use disorder (AUD) (Salvatore et al, 2015). Importantly, subjective responses to alcohol show heritability (Heath et al, 1999; Viken et al, 2003) and appear to satisfy additional endophenotype criteria (Ray and Heilig, 2013). Evidence further supports the association of candidate genes and indicators of latent genetic risk (i.e., family history of AUD) with laboratory responses to alcohol (Enoch, 2014; Hines et al, 2005; Ray and Heilig, 2013; Schuckit, 2009). Critical to the endophenotype concept is that the marker exists on a causal pathway between genotype and clinical syndrome, implying its status as a developmental precursor (Lenzenweger, 2013). Key findings from two research groups suggest that subjective responses to alcohol anticipate AUD risk, such that greater stimulant and hedonic responses (King et al, 2011; 2014) and lower subjective sedation (King et al, 2011; 2014; Schuckit, 1994; Schuckit and Smith, 2000) predict later heavy drinking and AUD symptoms. Notably, recent findings suggest that subjective responses relate to future AUD risk primarily among heavy drinkers, and when assessed at higher dosages (e.g., 0.8g/kg, King et al, 2011; 2014). Because prospective studies have been uncommon, additional work is needed to characterize subjective responses as neurodevelopmental traits pertinent to AUD etiology and clinical course.
Stimulant/hedonic and sedative effects of alcohol are presumed to reflect distinct neurobiological mechanisms (Hendler et al, 2013; Ray and Heilig, 2013) and appear to independently relate to event-level consumption (Wardell et al, 2015). Some theories propose that the ability of stimulant or sedative effects to discriminate AUD risk differs as a function of ascending versus descending blood alcohol concentration (BAC) limb (Newlin and Thomson, 1990), underscoring the importance of controlling BAC parameters when characterizing laboratory responses (Newlin and Renton, 2010; Quinn and Fromme, 2011). Because accurate control over BAC profiles is difficult in oral alcohol administration paradigms, owing largely to inter-individual differences in alcohol absorption and distribution kinetics, behavioral and neuroimaging studies have increasingly utilized intravenous alcohol administration as a means of maximizing pharmacokinetic control (e.g., Wetherill et al, 2012; Zimmermann et al, 2013).
Although subjective responses reflect an important component of AUD liability (Ray and Heilig, 2013), their assessment via self-report poses an inherent limitation for translational research, particularly given the lack of a direct analogue in animal models. Refinement of human self-administration paradigms could improve consilience between human and animal models (Leeman et al., 2010; Zimmermann et al, 2013), currently a noted research priority (Litten et al, 2015). Human laboratory studies linking subjective responses to self-administration phenotypes (e.g., de Wit and McCracken, 1990; Wardell et al, 2015) could further characterize mechanisms by which subjective responses relate to AUD risk. Ultimately, characterizing these associations within a developmental framework is important for positioning subjective responses as candidate endophenotypes.
Genetic associations with laboratory responses to alcohol have been evaluated largely using alcohol challenge paradigms (reviewed in Enoch, 2014). Of the few studies examining self-administration, some reported no association of FH with oral alcohol self-administration (de Wit and McCracken, 1990; Krishnan-Sarin et al, 2007). However, in an intravenous self-administration paradigm, young family history positive (FH+) participants achieved significantly higher breath alcohol concentration (BrAC) than family history negative (FN−) participants (Zimmermann et al, 2009). While candidate gene studies of laboratory self-administration are few, three studies examined the OPRM1 Asn40Asp (A118G) variant (rs1799971), a marker of particular interest given cross-species evidence implying its functional relevance for alcohol-related reward (for review see Heilig et al, 2011; Mague and Blendy, 2010; Ray et al, 2012). Whereas two studies using oral self-administration paradigms reported no association of OPRM1 with self-administration (Anton et al, 2012; Setiawan et al, 2011), an intravenous self-administration study found that Asp (118G allele) carriers achieved significantly higher peak BrAC relative to Asn (118A allele) homozygotes (Hendershot et al, 2014). Collectively, these findings imply that OPRM1 could serve as a useful model for studying genetic influences on alcohol responses across development, and that controlled intravenous paradigms might prove sensitive for detecting genetic associations with laboratory responses (Zimmermann et al, 2013).
The current study applied a short-term prospective human laboratory design to examine associations among background genetic factors, subjective responses and self-administration, and subsequent heavy drinking during late adolescence—a developmental period that remains under-studied in human laboratory research. A primary aim was to test prospective associations of subjective responses with laboratory self-administration among young heavy drinkers. We further examined whether laboratory self-administration predicted future heavy drinking, and tested indirect associations among background genetic factors, laboratory phenotypes, and future heavy drinking. Evidence for significant indirect associations of genetic factors with follow-up consumption via laboratory phenotypes would strengthen the argument for prioritizing laboratory paradigms in developmental and endophenotype studies of AUD.
Materials and Methods
Participants and recruitment
Participants (N=61, mean (M) age=19.89 years, SD=0.86) were recruited from University and community settings, and completed telephone screening to verify initial eligibility. Given evidence that prospective associations of laboratory responses with future consumption may be limited to heavy drinkers (King et al, 2011), participants were required to endorse heavy drinking (1+ episodes of ≥4 drinks for women or ≥5 drinks for men) in the prior month. Additional eligibility criteria included: age 19-21 (age was assessed in full years, irrespective of months since last birthday, and verified by photo identification), no treatment history or current efforts to reduce drinking, a Brief Michigan Alcohol Screening Test (MAST; Pokorny et al, 1972) score <10 (Conrod et al, 1998), a Fagerstrom Test for Nicotine Dependence (FTND; Heatherton et al, 1991) score <6, no prior adverse reactions to venipuncture, and no medical conditions contraindicating alcohol. A brief screener (Yokoyama et al, 2002) was used to exclude participants with likely aldehyde dehydrogenase deficiency, which would alter alcohol responses. The sample consisted predominantly of Caucasian participants (n=40); others reported Asian (n=6), East Indian (n=4), African (n=2), Native (n=1), mixed (n=2), or “other” (n=5) racial backgrounds. One participant endorsed Hispanic ethnicity without indicating race.
Recruitment efforts aimed to achieve a sample stratified on family history status (FH+ or FH−, defined below). To ensure that FH groups were comparable on key variables, group means/frequencies for age, sex, age of onset for regular drinking, and past-month heavy drinking were monitored during recruitment. In addition to ensuring that groups remained matched on age and sex, recruitment of FH- participants prioritized those whose drinking outcomes approximated mean values of the FH+ group (as assessed during the initial telephone screen), while excess FH- participants were excluded from laboratory sessions.
Eligible participants completed in-person visits, which included informed consent, height/weight measurements, verification of age and medical criteria, a toxicology screen, and a structured interview to confirm family history status. Baseline questionnaires were administered via computer, and participants provided saliva samples for genotyping. The present analyses include 40 participants from an initial report of this study (Hendershot et al, 2014). In addition to the larger sample (reflecting additional recruitment), the present study is distinct from the prior study in that it utilizes a second laboratory session (alcohol challenge) to test prospective associations between subjective responses and self-administration, uses multilevel modeling to analyze self-administration, includes a prospective follow-up component, and examines FH status in relation to these outcomes.
Laboratory Sessions
Eligible participants were scheduled for alcohol challenge (Session 1) and self-administration (Session 2) visits, occurring on average 4 weeks apart (M=26.70 days, SD=30.61, range 2-156). Despite variability in the number of days between sessions, most participants (92%) completed Session 2 within two months of Session 1. Time between sessions was not significantly correlated with any variables in the analyses (all ps>.05), and controlling for time did not alter any findings.
Upon arrival at each session participants consumed a standardized snack and provided a breath alcohol test, updated weight measurements, and a pregnancy test (females). Both sessions involved intravenous administration of ethanol in normal saline (6.0% v/v) using the Computer-assisted Alcohol Infusion System (CAIS; Ramchandani et al, 1999; Zimmermann et al, 2013). CAIS incorporates a physiologically-based pharmacokinetic model (Plawecki et al, 2008) to establish the infusion rates necessary to maintain an intended BAC profile, based on participant characteristics and in-session BrAC readings. Eighty-eight participants completed Session 1; of these, 61 completed Session 2 and had valid data for both sessions, representing the current sample. Of the remaining 27 participants, 14 were lost to follow-up or otherwise unavailable to complete Session 2, 6 were deemed ineligible to continue (due to difficulty with catheter insertion or intravenous procedures [4], failure to complete computerized tasks as instructed [1], or a change in medical eligibility status [1]). Five participants could not complete Session 2 due to pharmacy logistical barriers. Finally, two participants who completed Session 2 were excluded from analyses for not following task instructions (i.e., failing to engage in appreciable self-administration, as defined by a general decline in BrAC following a mandatory priming phase, followed by a BrAC reading of 0mg% during the self-administration phase).
Session 1
Session 1 (alcohol challenge) utilized an alcohol clamp paradigm (Ramchandani et al, 1999) to achieve a linear ascent from 0 to 80mg% in 20 minutes, after which the target BAC was maintained for 80 minutes. Because this paradigm essentially eliminates between-person differences in BAC slope, peak, and rate of change (Morzorati et al, 2002), Session 1 served to assess subjective responses under relatively uniform conditions of brain ethanol exposure. Participants completed serial assessments of subjective effects at baseline (pre-infusion) and 5 additional points (Hendershot et al, 2015). Participants’ maximum reported stimulation and sedation during the infusion (see Measures) served as primary outcomes.
Session 2
Session 2 involved a free-access intravenous self-administration paradigm (Zimmermann et al, 2008; 2009), in which participants submitted “drink” requests by pressing an electronic button. Each request triggered a 2.5-minute infusion calculated to achieve a linear BAC increment of 7.5mg%. Participants were instructed to self-administer at their discretion to achieve a pleasurable state of intoxication, while avoiding unpleasant effects. The session began with a priming phase, during which participants were prompted to self-administer four successive “drinks” over 10 minutes (target BrAC=30mg%). After a 5-minute wait, participants engaged in ad libitum self-administration from 15 to 120 minutes. If the model predicted that an ensuing button press would raise BAC beyond 100mg% a “bar closure” occurred, lasting until a subsequent drink request would not violate the BAC ceiling. Further details on this session are described elsewhere (Hendershot et al, 2014). Peak BrAC and within-session BrAC trajectories (based on breathalyzer readings) served as Session 2 outcomes.
Follow-up assessments
Participants completed an online follow-up assessment of alcohol consumption, scheduled 6 months after Session 1. Online surveys included standard drink charts to aid in reporting. Seven participants who did not complete the survey were excluded from prospective analyses, but did not differ on demographic variables, genotype, family history, subjective response variables, Session 2 peak BrAC, or baseline heavy drinking (all p>.05). Of those completing the follow-up, a mean of 29.56 (SD=5.15) weeks had elapsed from baseline. We controlled for this interval in all models predicting follow-up heavy drinking (see Data Analysis). Prior to the follow-up, a subset of the 61 participants (n=41) also completed a neuroimaging protocol (see Strang et al., 2015, for an initial report). Those completing the imaging component did not differ from other participants on the follow-up heavy drinking outcome (p=.53).
Measures
Alcohol Consumption
Similar to recent prospective research (King et al, 2011) frequency of heavy drinking served as the criterion follow-up outcome. Heavy drinking was assessed (at both baseline and 6-month follow-up) with the National Institute on Alcohol Abuse and Alcoholism (NIAAA) recommended alcohol questions (NIAAA, 2003). Participants reported the frequency of drinking 4+/5+ (women/men) drinks in a 2-hour period (0=never to 9=every day) in the past 90 days. To further characterize drinking outcomes, participants completed a 90-day Timeline Followback interview (Sobell and Sobell, 1992) and the 10-item Alcohol Use Disorders Identification Test (AUDIT) (Saunders et al, 1993) at the baseline assessment.
FH status
FH status was assessed with the Family History Assessment Module (FHAM), developed for the Collaborative Study on the Genetics of Alcoholism (COGA) (Rice et al, 1995). FH− participants reported no history of alcohol problems or dependence among first- or second-degree biological relatives. FH+ participants reported at least two biological relatives with alcohol dependence, including a requirement of a) ≥1 first-degree relatives, or b) a multigenerational (≥2 successive generations) history of dependence on the paternal side. Overall, 93% of FH+ participants met the former criterion (of which all but one reported dependence on the part of the father). The latter criterion was included because a multigenerational paternal history is a robust marker of response to alcohol (Conrod et al, 1998; Finn and Pihl, 1988). Consistent with many studies (Morean and Corbin, 2010), we excluded participants reporting maternal alcohol dependence to limit confounds related to fetal alcohol exposure. Familial dependence was still common among maternal relatives (M=0.74, SD=1.10 relatives), although less so than in paternal relatives (M=2.04, SD=1.19), in part reflecting this exclusion criterion.
Subjective Responses
Stimulant and sedative effects during Session 1 were assessed with the 14-item biphasic alcohol effects scale (BAES) (Martin et al, 1993). Items were presented on a computer monitor facing participants. The stimulation (e.g., energized, stimulated) and sedation (e.g., sedated, sluggish) subscales showed good internal consistency across all assessment points (α ranges: .83-.91 and .78-.88, respectively).
Genotyping
The OPRM1 A118G variant (rs1799971) was genotyped using DNA extracted from saliva samples (Oragene OG-500 DNA kit; DNA Genotek, Ottawa, ON), as previously reported (Hendershot et al, 2014). Genotype frequencies were (AA=45, GA=11, GG=5). Those with the G allele (GA/GG) were compared to AA homozygotes. For genotype differences in sample characteristics see Table 1.
Table 1.
Participant characteristics and variable descriptives by family history and OPRM1 genotype.
| Family History | OPRM1 | |||||||
|---|---|---|---|---|---|---|---|---|
| Negative (n=34) | Positive (n=27) | AA (n=45) | GA/GG (n=16) | |||||
| Categorical Variables | n (%) | n (%) | χ 2 | p | n (%) | n (%) | χ 2 | p |
| OPRM1: GA/GG | 10 (29%) | 6 (22%) | 0.40 | .53 | - | - | ||
| Sex: Female | 16 (47%) | 16 (59%) | 0.90 | .34 | 25 (56%) | 7 (44%) | 0.66 | .42 |
| Race: White | 19 (56%) | 21 (78%) | 3.20† | .07 | 33 (73%) | 7 (44%) | 4.58* | .03 |
| Current Cigarette Smoker | 9 (27%) | 7 (26%) | <0.01 | .96 | 12 (27%) | 4 (25%) | 0.02 | .90 |
| Completed 6 month follow up | 29 (85%) | 25 (93%) | 0.79 | .37 | 39 (87%) | 15 (94%) | 0.58 | .45 |
| Continuous Variables | M (SD) | M (SD) | t | p | M (SD) | M (SD) | t | p |
| Age | 19.82 (0.80) | 19.96 (0.94) | −0.63 | .53 | 19.91 (0.85) | 19.81 (0.91) | 0.39 | .70 |
| Age at first drink | 15.09 (1.53) | 14.67 (2.24) | 0.87 | .39 | 15.04 (1.76) | 14.50 (2.16) | 1.00 | .32 |
| Age became regular drinking | 17.33 (1.08) | 17.37 (1.50) | −0.11 | .91 | 17.45 (1.30) | 17.06 (1.18) | 1.06 | .30 |
| TLFB Baseline Heavy Drinking Frequency (90 days) | 13.44 (11.89) | 14.33 (9.70) | −0.32 | .75 | 13.31 (10.50) | 15.31 (12.20) | −.063 | .53 |
| NIAAA Baseline Heavy Drinking Frequency (90 days) | 3.65 (1.54) ≈9 daysa | 3.93 (1.90) ≈12 daysa | −0.63 | .53 | 3.60 (1.67) ≈9 daysa | 4.25 (1.73) ≈13 daysa | −1.32 | .19 |
| NIAAA 6 Month Heavy Drinking Frequency (90 days) | 2.69 (1.47) ≈5 daysa | 3.12 (2.15) ≈10 daysa | −0.87 | .39 | 2.72 (1.73) ≈6 daysa | 3.33 (1.99) ≈10 daysa | −1.12 | .27 |
| AUDIT Score | 9.41 (3.38) | 12.11 (6.02) | −2.22* | .03 | 10.24 (4.22) | 11.63 (6.45) | −0.97 | .34 |
| Peak Stimulation – Session 1 | 57.76 (16.17) | 65.13 (13.57) | −1.90† | .06 | 59.93 (16.78) | 64.10 (10.43) | −0.93 | .36 |
| Peak Sedation – Session 1 | 51.82 (16.34) | 53.32 (17.79) | −0.34 | .73 | 54.24 (16.37) | 47.53 (17.79) | 1.38 | .17 |
| Session 1 mean BrAC (mg%) during clamp period | 80.41 (1.41) | 80.05 (1.82) | 0.88 | .38 | 80.24 (1.45) | 80.26 (2.03) | −0.04 | .97 |
| Session 2 peak BrAC (mg%) | 80.94 (24.83) | 84.52 (24.80) | −0.56 | .58 | 78.51 (25.84) | 93.81 (17.10) | −2.20* | .03 |
Note.
Estimate of mean number of heavy drinking days in the past 90 days derived by assigning a representative value to each of the categorical response options for the NIAAA heavy drinking items. For example, “one day per month” = 1*3 months = 3 days; “one day per week” = 1*12 weeks = 12 days.
df = 1 for all chi-square tests; df = 59 for all t-tests, except for 6-month heavy drinking frequency (df=52) and age became regular drinker (df=58). †p<.10; * p<.05; ** p<.01.
Data Analysis
First, descriptive analyses compared OPRM1 and FH groups on demographic and drinking variables. To examine Session 2 self-administration as a function of OPRM1, FH and subjective responses (SR) from Session 1, we conducted multilevel modeling (MLM) using SPSS version 21. MLM is well suited to examining individual-level predictors of BrAC trajectories (i.e., changes in BrAC over time), which, in this paradigm, closely reflect self-administration behavior over the session. Eight scheduled BrAC measurements were modeled as the outcome in the MLM. The overall proportion of missing data across the 8 measurements was <2%; participants missing some BrAC data were retained through the use of MLM. Time point was coded with the post-priming BrAC measurement (15 min) as T=0 and an interval of 1 between each BrAC measurement. We first examined the shape of the average BrAC curve by entering successive polynomial functions (linear, quadratic, etc.) for the effect of time as predictors in the MLM, in order to examine the temporal progression of self-administration during the session. A random intercept and random slopes for all time effects were estimated to account for the variability across participants in BrAC curves.
Next, we examined individual-level risk factors (SR, OPRM1, FH), as moderators of the effect of time on BrAC in separate models. These variables were entered into the models along with their interactions with the time contrasts. SR variables were standardized across participants. Dummy coding was used for genotype (0=AA, 1=GA/GG) and family history (0=negative; 1=positive). A significant interaction between a predictor variable and a time contrast indicates that the effect of time on BrAC depends on levels of the predictor variable. Also, because the interactions are included in the model, the main effect of time represents a conditional effect (i.e., the effect of time when the moderator is held constant at zero). Significant interactions were probed using simple slopes analysis (Aiken & West, 1991). This approach involves shifting the reference point of the moderator (i.e., the zero value) to obtain the conditional effect of time at different levels of the moderator. We used the standard approach of examining the conditional effects of time at high (M+1SD) and low (M−1SD) values of SR variables, as well conditioned on each of the genotype and FH groups (Aiken & West, 1991; Preacher, Curran, Bauer, 2006).
Next, we tested the hypothesis that self-administration (indexed by Session 2 peak BrAC) would mediate the relationship between individual-level risk factors (FH, OPRM1) and heavy drinking at 6 months, using the SPSS PROCESS macro (Hayes, 2013). This regression-based approach tests indirect pathways through intervening variables (i.e., mediation) by calculating the product of the coefficients for the paths comprising the indirect associations. Bootstrapping is used to calculate confidence intervals for the estimates of the indirect associations. A statistically significant indirect association (i.e., 95% confidence interval does not contain zero) provides support for the mediational role of the intervening variable. This approach allows for examination of an indirect association without the pre-condition that the independent variable is significantly associated with the outcome variable. Particularly longitudinal studies, it is often the case that the effect of a distal independent variable on the outcome variable is small, but this does not necessarily preclude the presence of a mediation effect that can be detected using the product of coefficients method (e.g., Shrout and Bolger, 2002).
We also examined sequential multiple-mediator pathways to investigate 4-variable pathways (FH/OPRM1 to Session 1 SR to Session 2 BrAC to 6-month heavy drinking). Baseline heavy drinking and time between baseline and follow-up assessments were included as covariates in all models, and all variables were standardized prior to analyses.
Results
Descriptive Statistics
Table 1 shows the model variables, participant characteristics, and BrAC outcomes by genotype and FH. Participants completing only one (versus both) lab sessions did not differ significantly on sex, race, FH status, OPRM1 genotype, AUDIT scores, or Session 1 subjective response outcomes (p > .05), but reported lower heavy drinking frequency (M=2.92, SD=1.55) compared to those completing both sessions (M=3.75, SD=1.76), t(86)=−2.05, p=.043. Mean BrAC values across Session 1 closely approximated the 80mg% target. While FH+ participants reported marginally greater peak stimulation relative to FH− participants, genotype was not associated with Session 1 subjective responses. Additionally, FH status was not associated with Session 2 peak BrAC. However, the OPRM1 GA/GG group achieved significantly higher peak BrAC relative to the AA group.
Roughly half (32) of participants reached a projected BAC level high enough to initiate a “bar closure.” The likelihood of at least one closure did not differ based on FH, sex, or genotype. However, the total number of bar closures was significantly higher in the OPRM1 GA/GG group (vs. AA participants, p=.04) and in men (vs. women; p=.02), suggesting that these two groups attempted to surpass the BAC ceiling more often than their counterparts.
Although the OPRM1 GA/GG group had a significantly higher proportion of non-white participants relative to the AA group (Table 1), race was not significantly related to baseline or follow-up consumption measures, Session 1 stimulation/sedation, or Session 2 peak BrAC (all ps> .05). In no instance did covarying for race alter any associations between OPRM1 and other variables. Still, race (Caucasian vs. non-Caucasian) was included as a covariate in all analyses involving OPRM1.
Multilevel Models of Session 2 Self-Administration
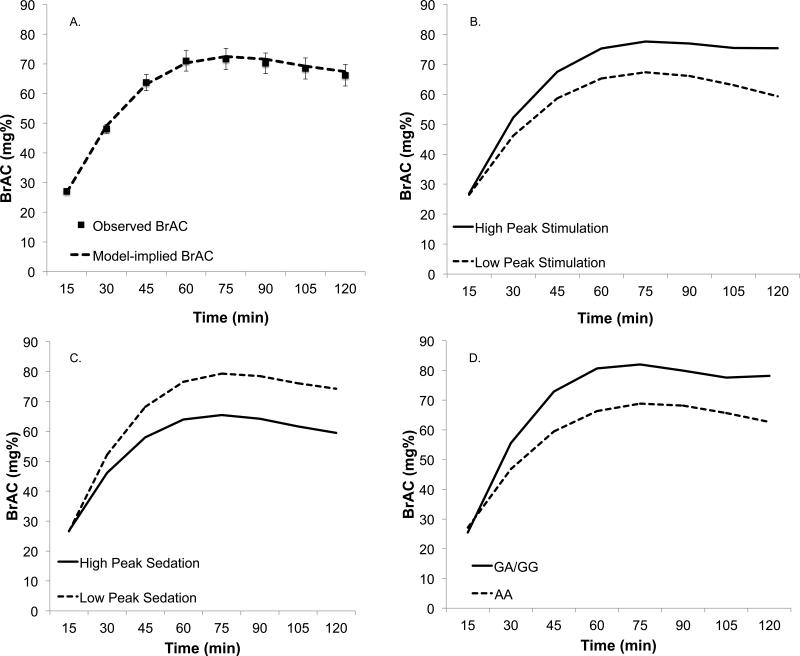
Results of the multilevel model of the unconditional changes in BrAC during Session 2 are presented in Table 2. The fixed effect for the fourth-order polynomial time was not significant (p=.76) and was removed from the model. Thus, the final MLM modeled the average BrAC curve as a cubic trend. Because time was coded with t=0 referring to the beginning of the self-administration period, the linear effect of time suggests a statistically significant initial increase in BrAC, the quadratic trend suggests that increases in BrAC gradually slowed over time and then began to decline, and the cubic trend suggests that BrAC significantly leveled off toward the end of the session (i.e., the decline was significantly less steep than would be expected from a quadratic trend alone). Figure 1a shows the model-implied and observed mean BrAC curve during the session.
Table 2.
Multilevel models (MLM) examining change in observed BrAC during intravenous self-administration (Session 2) as a function of time and between-person moderators (subjective stimulant and sedative response to alcohol during Session 1 and OPRM1 genotype).
| Fixed Effects | B | SE | t | p |
|---|---|---|---|---|
| Model 1: Unconditional Effect of Time | ||||
| Time contrasts (Level 1) | ||||
| Linear | 27.37** | 1.78 | 15.42 | <.001 |
| Quadratic | −5.19** | 0.44 | −11.90 | <.001 |
| Cubic | 0.30** | 0.04 | 7.47 | <.001 |
| Model 2: Subjective Response | ||||
| Between-person (Level 2) | ||||
| Peak Stimulation (session 1) | 0.21 | 0.94 | 0.23 | .821 |
| Peak Sedation (session 1) | 0.08 | 0.94 | 0.09 | .932 |
| Interactions with Time | ||||
| Peak Stimulation*Linear | 3.64* | 1.70 | 2.15 | .034 |
| Peak Stimulation*Quadratic | −0.92* | 0.44 | −2.11 | .036 |
| Peak Stimulation*Cubic | 0.08† | 0.04 | 1.93 | .055 |
| Peak Sedation*Linear | −3.73* | 1.69 | −2.20 | .030 |
| Peak Sedation*Quadratic | 0.65 | 0.43 | 1.50 | .134 |
| Peak Sedation*Cubic | −0.04 | 0.04 | −0.94 | .350 |
| Model 3: OPRM1 Genotype | ||||
| Between-person (Level 2) | ||||
| Race (0=white; 1=nonwhite) | 0.02 | 0.98 | 0.02 | .985 |
| Genotype (0=AA; 1=GA/GG) | −1.66 | 2.21 | −0.75 | .454 |
| Interactions with Time | ||||
| Race*Linear | 0.02 | 1.76 | 0.01 | .992 |
| Race*Quadratic | 0.12 | 0.44 | 0.28 | .779 |
| Race*Cubic | −0.01 | 0.04 | −0.35 | .727 |
| Genotype*Linear | 13.78** | 3.96 | 3.48 | .001 |
| Genotype*Quadratic | −3.66** | 0.99 | −3.70 | <.001 |
| Genotype*Cubic | 0.30** | 0.09 | 3.20 | .002 |
Note. Unstandardized parameter estimates shown. Estimates of fixed effects are shown; random intercepts and random slopes for all time contrasts were estimated in all models but are not shown in the table to conserve space. Time contrasts are estimated at the within-person level (level 1) and subjective response and genotype are modeled at the between-person level (level 2). Time was coded with the post-priming BrAC measurement (15 min) as T=0 and an interval of 1 between each successive BrAC measurement. Peak stimulation and peak sedation are grand-mean centered and standardized across participants (i.e., z-scored); †p<.10; *p<.05; **p<.01.
Figure 1.
Results of the multilevel models (MLM) of change in BrAC over the self-administration session (Session 2). Panel A shows the unconditional effects of time derived from the MLM (i.e., the model-implied BrAC) with observed mean BrAC readings superimposed (standard errors in error bars). Panel B shows the simple slopes for the effects of time at high (M+1SD) and low (M−1SD) levels of peak stimulation during session 1. Panel C shows the simple slopes for the effects of time at high (M+1SD) and low (M−1SD) levels of peak sedation during session 1. Panel D shows the simple slopes for the effects of time conditioned on each of the genotype groups. Race is included as a covariate in the genotype model (Panel D).
We next entered Session 1 stimulant and sedative responses together as predictors of Session 2 BrAC, as well as their interactions with all time trends. Peak stimulation significantly interacted with the linear, quadratic, and cubic time trends (Table 2), suggesting that Session 1 peak stimulation was a significant predictor of BrAC change during Session 2. Relative to individuals reporting lower peak stimulation, those reporting greater peak stimulation showed a steeper initial increase in BrAC (significant interaction with the linear time trend), less of a decline in BrAC during the second half of the session (significant interaction with the quadratic time trend), and a leveling off and slight increase in BrAC toward the end of the session (significant interaction with the cubic time trend) (Figure 1b).
Sedative response to alcohol significantly interacted with the linear effect of time only (Table 2). This indicates that lower peak sedation during Session 1 was associated with a steeper initial rate of increase in BrAC during Session 2 (interaction with linear trend), but that the shape of the curve later in the session was not different, as indicated by the non-significant interactions with the quadratic and cubic trends (see Figure 1c). Controlling for Session 1 baseline ratings of stimulation and sedation did not affect the results of the MLM models, nor were baseline ratings significantly related to Session 2 BrAC.
OPRM1 genotype interacted significantly with all three time trends (Table 2). Relative to AA homozygotes, the GA/GG group showed a steeper initial increase in BrAC (interaction with linear trend), less of a slowing in BrAC increases as time progressed (interaction with quadratic trend) and less of a decline (and indeed a slight increase) in BrAC toward the end of the session (interaction with cubic trend; see Figure 1d).
Indirect Associations with Follow-up Heavy Drinking
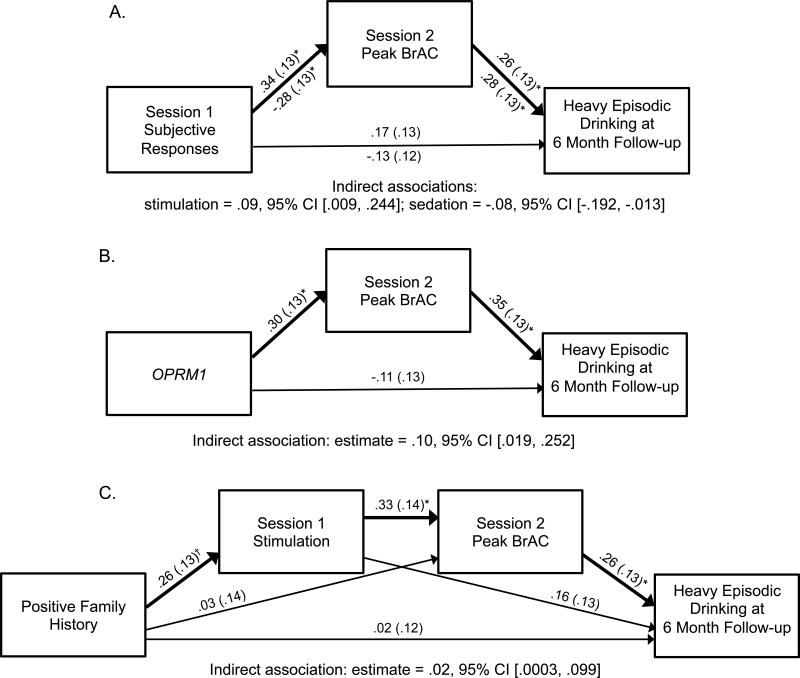
Results of indirect association analyses are shown in Figure 2. Session 1 peak stimulation significantly predicted greater Session 2 peak BrAC, which in turn was a significant predictor of greater heavy drinking at 6 months (Figure 2a). Moreover, the indirect association from peak stimulation to heavy drinking at 6 months (mediated via Session 2 peak BrAC) was statistically significant. Peak sedation during Session 1 showed a negative relationship with peak BrAC during Session 2, which in turn predicted heavy drinking at 6 months. This indirect association was statistically significant. Results of the mediation model for genotype (Figure 2b) showed that OPRM1 GA/GG status related to greater peak BrAC, which in turn predicted more frequent heavy drinking at 6-month follow-up; this indirect association was statistically significant. However, FH did not predict peak BrAC (p>.05), nor was the indirect association from FH to heavy drinking at 6 months statistically significant (95% CI contains zero).
Figure 2.
Models of the indirect associations from individual-level risk factors to heavy episodic drinking at 6 month follow up mediated via peak BrAC during the self-administration (Session 2). Standardized coefficients are shown with standard errors in parentheses. Bolded arrows highlight the indirect associations that are tested. Panel A shows the indirect associations from peak subjective responses during session 1, with coefficients for the stimulation model above the arrows and coefficients for the sedation model below the arrows. Panel B shows the indirect association from OPRM1 (rs1799971) genotype (0=AA, 1=GA/GG), while controlling for race. Panel C shows the 4-variable indirect association from family history (0=negative, 1=positive) to heavy drinking at 6 months mediated through both Session 1 peak stimulation and Session 2 peak BrAC. All models covary for heavy episodic drinking at baseline and number of weeks between baseline and follow up assessments. †p<.10; *p<.05; **p<.01.
Given that FH was marginally associated with Session 1 peak stimulation, we tested a 4-variable model including FH, stimulation, peak BrAC, and follow-up heavy drinking (Figure 2c). FH+ status was a marginally significant predictor of greater peak stimulation, which in turn related to significantly higher peak BrAC, which in turn related to significantly greater heavy drinking at follow-up. This 4-variable indirect pathway was statistically significant. Of note, none of the direct associations between background factors and 6-month heavy drinking frequency were statistically significant in these models (see Figure 2), or in separate regression models that excluded the mediator (ps > .05).
Discussion
Subjective responses to alcohol are proposed as an endophenotypic marker of AUD liability (Morean and Corbin, 2010; Quinn and Fromme, 2011; Ray and Heilig, 2013), as well as a potential index of AUD progression (Bujarski et al, 2015; King et al, 2015). Although these perspectives call for longitudinal research, prospective human laboratory studies have been rare. Additionally, self-administration paradigms—which have the capacity to help characterize associations of subjective responses with consumption—are infrequently used in this literature. This study applied a prospective human laboratory design to examine associations of subjective responses with subsequent laboratory self-administration and self-reported heavy drinking. Peak stimulation and sedation during a highly controlled alcohol challenge independently predicted subsequent laboratory self-administration, as indexed by maximum BrAC and within-session BrAC trajectories. These results coincide with evidence that stimulation and sedation during alcohol challenge predict self-reported consumption and AUD symptoms prospectively (King et al, 2011; 2014), providing laboratory-based support for higher event-level alcohol exposure as a potential mediator of these associations. In particular, peak BrAC accounted for significant indirect associations between laboratory subjective responses and follow-up heavy drinking. The present study is the first, to our knowledge, to link laboratory self-administration with prospective heavy drinking.
Results concerning FH status were only partly consistent with prior research. FH+ participants reported marginally greater Session 1 stimulation, a finding inconsistent with the low level of response (LLR) theoretical model (Schuckit, 1994), and partly consistent with the differentiator model (Newlin and Thomson, 1990). This result is less readily interpreted in light of the modified differentiator model (King et al, 2011), because research supporting this framework emphasizes heavy drinking status (rather than FH) as a risk marker, and has reported no association of FH with subjective responses (King et al, 2011). Despite some prior evidence that FH+ participants reported greater stimulant effects during intravenous alcohol challenge (Morzorati et al, 2002), similar studies reported no FH group differences (Kerfoot et al, 2013; Wetherill et al, 2012). Notably, meta-analytic findings suggested that FH+ participants show reduced subjective responses, both across subjective response domains and across BAC limb (Quinn and Fromme, 2011). However, significant FH group differences appeared specific to men, and most studies used assessment instruments that do not capture hedonic effects. In sum, evidence that FH relates to subjective responses remains somewhat mixed, although studies involving physiological measures of alcohol reward have provided more consistent evidence that FH+ participants show enhanced stimulant responses (e.g., Conrod et al, 1998; see Newlin and Renton, 2010).
Despite some evidence for FH differences in reported stimulation, FH status did not significantly relate to self-administration. Similar results were reported in studies using oral self-administration paradigms (de Wit and McCracken, 1990; Krishnan-Sarin et al, 2007). In contrast, during free-access intravenous self-administration, FH+ young adults achieved higher peak BrAC relative to FH− participants (Zimmermann et al, 2009). However, this difference was evident only following a practice session, which was not a feature of the present study. Notably, the present study utilized a lower BrAC ceiling (100mg%) compared to the study by Zimmermann and colleagues (120mg%). Nonetheless, compared to participants in the prior study, those in the current study achieved relatively higher mean peak BrAC. Therefore, the possibility that FH group differences were obscured by a ceiling effect cannot be ruled out, particularly given that half of the participants in the present study reached the BrAC limit.
The lack of OPRM1 genotype differences in Session 1 subjective responses is inconsistent with prior studies using intravenous alcohol challenge (Ray and Hutchison, 2004; 2007; Ray et al, 2013). Nonetheless, GA/GG participants achieved significantly higher peak BrAC (Session 2), a finding that remained consistent after expanding the sample size following our prior report (Hendershot et al, 2014). Moreover, multilevel modeling revealed significant genotype differences in BrAC trajectories across the session, indicating greater initial escalation (and sustained elevations) in BrAC among GA/GG participants relative to AA homozygotes. Notably, these differences in event-level self-administration appear consistent with reports of prior-day consumption during an ecological momentary assessment study (Ray et al., 2010), and with non-human primate studies examining the OPRM1 C77G variant, considered functionally analogous to OPRM1 A118G (reviewed in Heilig et al., 2011).
Explanations for OPRM1 group differences in self-administration, but not subjective responses, are not necessarily clear. Notably, one study reported enhanced striatal dopamine response during intravenous alcohol challenge in men with the 118G variant, despite no substantial genotype differences in subjective responses (Ramchandani et al, 2011). One implication is that objective measures of alcohol-related reward may be more sensitive for detecting genetic associations relative to self-report measures. A related possibility is that genotype differences in self-administration may reflect aspects of alcohol-related reward or motivation that were not adequately captured by the measures used in this study. An additional consideration is the younger age of this sample relative to prior studies of OPRM1 and subjective responses. To the extent that genotype differences in subjective responses may arise from differences in drug sensitization or other neurodevelopmental processes, genotype differences in subjective responses may be expected to manifest only following a certain degree of alcohol exposure. Presently, the lack of developmental research in this area (Hendershot et al, 2015; Morean and Corbin, 2010) makes it difficult to infer directional associations between changes in subjective responses and consumption patterns over time, or evaluate genetic moderators thereof.
This study also examined indirect associations between latent (FH) and measured (OPRM1) genetic factors and future drinking via laboratory phenotypes. Path models supported significant indirect associations of FH+ status (via Session 1 stimulation and Session 2 peak BrAC) and OPRM1 GA/GG status (via peak BrAC) with follow-up heavy drinking. Of note, neither genetic factors nor subjective responses showed significant direct associations with follow-up heavy drinking. This finding suggests that self-administration phenotypes (in this instance, maximum BrAC during free-access conditions) may be useful for modeling associations between background risk factors and prospective AUD risk. These findings also support the notion that self-administration phenotypes can anticipate risky drinking patterns in youth (Zimmermann et al, 2013). Overall, greater efforts to model self-administration could help to clarify mechanisms by which subjective responses relate to AUD risk (e.g., Wardell et al, 2015).
Limitations of this study should be considered, including the moderate sample size and the short prospective follow-up period. An important limitation of the current study was the lack of a placebo manipulation, as discussed in detail elsewhere (Hendershot et al, 2015; Wardell et al, 2015). The reduced external validity of intravenous administration can also be considered a limitation; however, the high experimental precision afforded by these paradigms is also a distinct advantage for human laboratory studies (Zimmermann et al, 2013). A potential limitation is that our follow-up assessment of heavy drinking frequency, while based on a valid measure (NIAAA, 2003), was assessed via remote methods, resulting in less nuanced assessment relative to interview-based methods. Additionally, our prospective analysis did not include an assessment of AUD symptoms, which have also been associated with prior laboratory responses (King et al, 2011, 2014). Although not a limitation, it should also be noted that participants, on average, reported declines in heavy drinking frequency from baseline to follow-up. Therefore, higher BrAC during self-administration predicted more minimal reductions (rather than escalations) in heavy drinking. Finally, because this study involved targeted recruitment of young heavy drinkers, conclusions cannot necessarily be extrapolated to other populations.
Evidence for prospective associations of laboratory responses to alcohol with AUD liability (e.g., King et al, 2011; 2014; 2015; Schuckit and Smith, 2000) is one important consideration in determining the viability of subjective responses as endophenotypes. However, further developmental research is necessary to inform this question and to better characterize responses to alcohol as neurodevelopmental phenomena. A key aim will be to study developmental changes in alcohol response phenotypes, and their relation with drinking trajectories and emergence of clinical symptoms, using a neurodevelopmental framework (e.g., Casey et al, 2014). Given barriers to studies involving alcohol exposure in adolescence, alternative approaches (e.g., Miranda et al, 2014) will be necessary to complement laboratory-based methods for characterizing alcohol sensitivity across development.
Acknowledgements
This study was supported by a grant from ABMRF/The Foundation for Alcohol Research (CH). The authors also acknowledge support from Canadian Institutes of Health Research grants MOP-119444, MSH-130189 (CH) and MFE-140817 (JW); the Canada Foundation for Innovation and Ministry of Research and Innovation (CH), the Ontario Mental Health Foundation (CH); and the NIAAA Division of Intramural Clinical and Biological Research (VR). This research was supported, in part, by funding from the Canada Research Chairs program. The authors thank Sean O'Connor, Martin Plawecki and Victor Vitvitskiy at the Indiana Alcohol Research Center (NIH P60 AA007611) for technical support, Ariel Graff for his assistance with medical oversight, and Mike Markovich for his assistance with data collection. The authors declare no conflicts of interest.
Footnotes
Author Contributions
CH planned and carried out the study and wrote the Introduction, Materials and Methods, and Discussion sections. JW conducted the data analysis and wrote the Analysis Plan and Results sections. MM assisted with data collection and manuscript preparation. VR assisted with the development of the protocol, provided support with data collection, and provided critical review of the manuscript. All authors contributed to and approved the final manuscript.
References
- Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Sage; Newbury Park, CA: 1991. [Google Scholar]
- Anton RF, Voronin KK, Randall PK, Myrick H, Tiffany A. Naltrexone modification of drinking effects in a subacute treatment and bar-lab paradigm: influence of OPRM1 and dopamine transporter (SLC6A3) genes. Alcohol Clin Exp Res. 2012;36:2000–2007. doi: 10.1111/j.1530-0277.2012.01807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burjarski S, Hutchison KE, Prause N, Ray LA. Functional significance of subjective response to alcohol across levels of alcohol exposure. Addict Biol. 2015 doi: 10.1111/adb.12293. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Casey BJ, Oliveri ME, Insel T. A neurodevelopmental perspective on the research domain criteria (RDoC) framework. Biol Psychiatry. 2014;76:350–353. doi: 10.1016/j.biopsych.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Conrod PJ, Pihl RO, Vassileva J. Differential sensitivity to alcohol reinforcement in groups of men at risk for distinct alcoholism subtypes. Alcohol Clin Exp Res. 1998;22:585–597. doi: 10.1111/j.1530-0277.1998.tb04297.x. [DOI] [PubMed] [Google Scholar]
- de Wit H, McCracken SH. The reinforcing and subjective effects of ethanol in males with or without an alcoholic first degree relative. Alcohol Clin Exp Res. 1990;14:63–70. doi: 10.1111/j.1530-0277.1990.tb00448.x. [DOI] [PubMed] [Google Scholar]
- Enoch MA. Genetic influences on response to alcohol and response to pharmacotherapies for alcoholism. Pharmacol Biochem Behav. 2014;123:12–24. doi: 10.1016/j.pbb.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn PR, Pihl RO. Risk for alcoholism: A comparison between two different groups of sons of alcoholics on cardiovascular reactivity and sensitivity to alcohol. Alcohol Clin Exp Res. 1988;12:742–747. doi: 10.1111/j.1530-0277.1988.tb01338.x. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. Guilford Press; New York, NY: 2013. [Google Scholar]
- Heath AC, Madden PAF, Bucholz KK, Dinwiddie SH, Slutske WS, Bierut LJ, et al. Genetic differences in alcohol sensitivity and the inheritance of alcoholism risk. Psychol Med. 1999;29:1069–1081. doi: 10.1017/s0033291799008909. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heilig M, Goldman D, Berrettini W, O'Brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nat Rev Neurosci. 2011;12:670–684. doi: 10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot CS, Claus ED, Ramchandani VA. Associations of OPRM1 A118G and alcohol sensitivity with intravenous alcohol self-administration in young adults. Addict Biol. 2014 doi: 10.1111/adb.12165. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot CS, Wardell JD, Strang NM, Markovich MSD, Claus ED, Ramchandani VA. Application of an alcohol clamp paradigm to examine inhibitory control, subjective responses, and acute tolerance in late adolescence. Exp Clin Psychopharmacol. 2015;23:147–158. doi: 10.1037/pha0000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendler RA, Ramchandani VA, Gilman J, Hommer DW. Stimulant and sedative effects of alcohol. Curr Top Behav Neurosci. 2013;13:489–509. doi: 10.1007/7854_2011_135. [DOI] [PubMed] [Google Scholar]
- Hines LM, Ray L, Hutchison K, Tabakoff B. Alcoholism: The dissection of endophenotypes. Dialogues Clin Neurosci. 2005;7:153–163. doi: 10.31887/DCNS.2005.7.2/lhines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerfoot K, Pittman B, Ralevski E, Limoncelli D, Koretski J, Newcomb J, et al. Effects of family history of alcohol dependence on the subjective response to alcohol using the intravenous alcohol clamp. Alcohol Clin Exp Res. 2013;37:2011–2018. doi: 10.1111/acer.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry. 2011;68:389–399. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Hasin D, O'Connor SJ, McNamara PJ, Cao D. A prospective 5-year re-examination of alcohol response in heavy drinkers progressing in alcohol use disorder. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.05.007. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, McNamara PJ, Hasin DS, Cao D. Alcohol challenge responses predict future alcohol use disorder symptoms: A 6-year prospective study. Biol Psychiatry. 2014;75:798–806. doi: 10.1016/j.biopsych.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Krystal JH, Shi J, Pittman B, O'Malley SS. Family history of alcoholism influences naltrexone-induced reduction in alcohol drinking. Biol Psychiatry. 2007;62:694–697. doi: 10.1016/j.biopsych.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Leeman RF, Heilig M, Cunningham CL, Stephens DN, Duka T, O'Malley SS. Ethanol consumption: How should we measure it? Achieving consilience between human and animal phenotypes. Addict Biol. 2010;15:109–124. doi: 10.1111/j.1369-1600.2009.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzenweger MF. Thinking clearly about the endophenotype–intermediate phenotype–biomarker distinctions in developmental psychopathology research. Dev Psychopathol. 2013;25:1347–1357. doi: 10.1017/S0954579413000655. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Falk DE, Reilly M, Fertig JB, Koob GF. Heterogeneity of alcohol use disorder: Understanding mechanisms to advance personalized treatment. Alcohol Clin Exp Res. 2015;39:579–584. doi: 10.1111/acer.12669. [DOI] [PubMed] [Google Scholar]
- Mague SD, Blendy JA. OPRM1 SNP (A118G): Involvement in disease development, treatment response, and animal models. Drug Alcohol Depend. 2010;108:172–182. doi: 10.1016/j.drugalcdep.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the biphasic alcohol effects scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Miranda R, Jr., Monti PM, Ray L, Treloar HR, Reynolds EK, Ramirez J, et al. Characterizing subjective responses to alcohol among adolescent problem drinkers. J Abnorm Psychol. 2014;123:117–129. doi: 10.1037/a0035328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, Corbin WR. Subjective response to alcohol: A critical review of the literature. Alcohol Clin Exp Res. 2010;34:385–395. doi: 10.1111/j.1530-0277.2009.01103.x. [DOI] [PubMed] [Google Scholar]
- Morzorati SL, Ramchandani VA, Flury L, Li TK, O'Connor S. Self-reported subjective perception of intoxication reflects family history of alcoholism when breath alcohol levels are constant. Alcohol Clin Exp Res. 2002;26:1299–1306. doi: 10.1097/01.ALC.0000025886.41927.83. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism Task Force on Recommended Questions [23 December, 2015];National Council on Alcohol Abuse and Alcoholism Recommended Sets of Alcohol Consumption Questions, October 15–16, 2003. 2003 Available at: http://www.niaaa.nih.gov/research/guidelines-and-resources/recommended-alcohol-questions.
- Newlin DB, Renton RM. High risk groups often have higher levels of alcohol response than low risk: The other side of the coin. Alcohol Clin Exp Res. 2010;34:199–202. doi: 10.1111/j.1530-0277.2009.01081.x. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: A critical review and analysis. Psychol Bull. 1990;108:383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- Plawecki MH, Han JJ, Doerschuk PC, Ramchandani VA, O'Connor SJ. Physiologically based pharmacokinetic (PBPK) models for ethanol. IEEE Trans Biomed Eng. 2008;55:2691–2700. doi: 10.1109/TBME.2008.919132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokorny AD, Miller BA, Kaplan HB. The brief MAST: A shortened version of the Michigan Alcoholism Screening Test. Am J Psychiatry. 1972;129:342–345. doi: 10.1176/ajp.129.3.342. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat. 2006;31:437–448. [Google Scholar]
- Quinn PD, Fromme K. Subjective response to alcohol challenge: A quantitative review. Alcohol Clin Exp Res. 2011;35:1759–1770. doi: 10.1111/j.1530-0277.2011.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani VA, Bolane J, Li T-K, O'Connor S. A physiologically-based pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcohol Clin Exp Res. 1999;23:617–623. [PubMed] [Google Scholar]
- Ramchandani VA, Umhau J, Pavon FJ, Ruiz-Velasco V, Margas W, Sun H, et al. A genetic determinant of the striatal dopamine response to alcohol in men. Mol Psychiatry. 2011;16:809–817. doi: 10.1038/mp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Barr CS, Blendy JA, Oslin D, Goldman D, Anton RF. The role of the Asn40Asp polymorphism of the mu opioid receptor gene (OPRM1) on alcoholism etiology and treatment: A critical review. Alcohol Clin Exp Res. 2012;36:385–394. doi: 10.1111/j.1530-0277.2011.01633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Mackillop J, Courtney KE, Monti PM, Miotto K. Subjective response to alcohol among alcohol-dependent individuals: Effects of the mu-opioid receptor (OPRM1) gene and alcoholism severity. Alcohol Clin Exp Res. 2013;37:E116–E124. doi: 10.1111/j.1530-0277.2012.01916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Heilig MA. Subjective Responses to Alcohol as an Endophenotype: Implications for Alcoholism Etiology and Treatment Development. In: MacKillop J, Munafo M, editors. Genetic Influences on Addiction. MIT Press; Cambridge, MA: 2013. pp. 97–120. [Google Scholar]
- Ray LA, Hutchison KE. A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol Clin Exp Res. 2004;28:1789–1795. doi: 10.1097/01.alc.0000148114.34000.b9. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: A double-blind placebo-controlled study. Arch Gen Psychiatry. 2007;64:1069–1077. doi: 10.1001/archpsyc.64.9.1069. [DOI] [PubMed] [Google Scholar]
- Ray LA, Miranda R, Jr., Tidey JW, McGeary JE, MacKillop J, Gwaltney CJ, et al. Polymorphisms of the mu-opioid receptor and dopamine D4 receptor genes and subjective responses to alcohol in the natural environment. J Abnorm Psychol. 2010;119:115–125. doi: 10.1037/a0017550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Salvatore JE, Gottesman II, Dick DM. Endophenotypes for alcohol use disorder: An update on the field. Curr Addict Rep. 2015;2:76–90. doi: 10.1007/s40429-015-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. An overview of genetic influences in alcoholism. J Subst Abuse Treat. 2009;36:S5–14. [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. The relationships of a family history of alcohol dependence, a low level of response to alcohol and six domains of life functioning to the development of alcohol use disorders. J Stud Alcohol Drugs. 2000;61:827–835. doi: 10.15288/jsa.2000.61.827. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychol Methods. 2002;7:422–445. [PubMed] [Google Scholar]
- Setiawan E, Pihl RO, Cox SM, Gianoulakis C, Palmour RM, Benkelfat C, et al. The effect of naltrexone on alcohol's stimulant properties and self-administration behavior in social drinkers: Influence of gender and genotype. Alcohol Clin Exp Res. 2011;35:1134–1141. doi: 10.1111/j.1530-0277.2011.01446.x. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MBM. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten R, Allen JP, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Springer; New York: 1992. pp. 41–72. [Google Scholar]
- Strang NM, Claus ED, Ramchandani VA, Graff-Guerrero A, Boileau I, Hendershot CS. Dose-dependent effects of intravenous alcohol administration on cerebral blood flow in young adults. Psychopharmacology (Berl) 2015;232:733–744. doi: 10.1007/s00213-014-3706-z. [DOI] [PubMed] [Google Scholar]
- Viken RJ, Rose RJ, Morzorati SL, Christian JC, Li TK. Subjective intoxication in response to alcohol challenge: Heritability and covariation with personality, breath alcohol level, and drinking history. Alcohol Clin Exp Res. 2003;27:795–803. doi: 10.1097/01.ALC.0000067974.41160.95. [DOI] [PubMed] [Google Scholar]
- Wardell JD, Ramchandani VA, Hendershot CS. A multilevel structural equation model of within- and between-person associations among subjective responses to alcohol, craving, and laboratory alcohol self-administration. J Abnorm Psychol. 2015;124:1050–1063. doi: 10.1037/abn0000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill L, Morzorati SL, Foroud T, Windisch K, Darlington T, Zimmerman US. Subjective perceptions associated with the ascending and descending slopes of breath alcohol exposure vary with recent drinking history. Alcohol Clin Exp Res. 2012;36:1050–1057. doi: 10.1111/j.1530-0277.2011.01642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama A, Watanabe H, Fukuda H, Haneda T, Kato H, Yokoyama T, et al. Multiple cancers associated with esophageal and oropharyngolaryngeal squamous cell carcinoma and the aldehyde dehydrogenase-2 genotype in male Japanese drinkers. Cancer Epidemiol Biomarkers Prev. 2002;11:895–900. [PubMed] [Google Scholar]
- Zimmermann US, Mick I, Laucht M, Vitvitskiy V, Plawecki MH, Mann KF, et al. Offspring of parents with an alcohol use disorder prefer higher levels of brain alcohol exposure in experiments involving computer-assisted self-infusion of ethanol (CASE). Psychopharmacology (Berl) 2009;202:689–697. doi: 10.1007/s00213-008-1349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann US, Mick I, Vitvitskiy V, Plawecki MH, Mann KF, O'Connor S. Development and pilot validation of computer-assisted self-infusion of ethanol (CASE): A new method to study alcohol self-administration in humans. Alcohol Clin Exp Res. 2008;32:1321–1328. doi: 10.1111/j.1530-0277.2008.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann US, O'Connor S, Ramchandani VA. Modeling alcohol self-administration in the human laboratory. Curr Top Behav Neurosci. 2013;13:315–353. doi: 10.1007/7854_2011_149. [DOI] [PubMed] [Google Scholar]