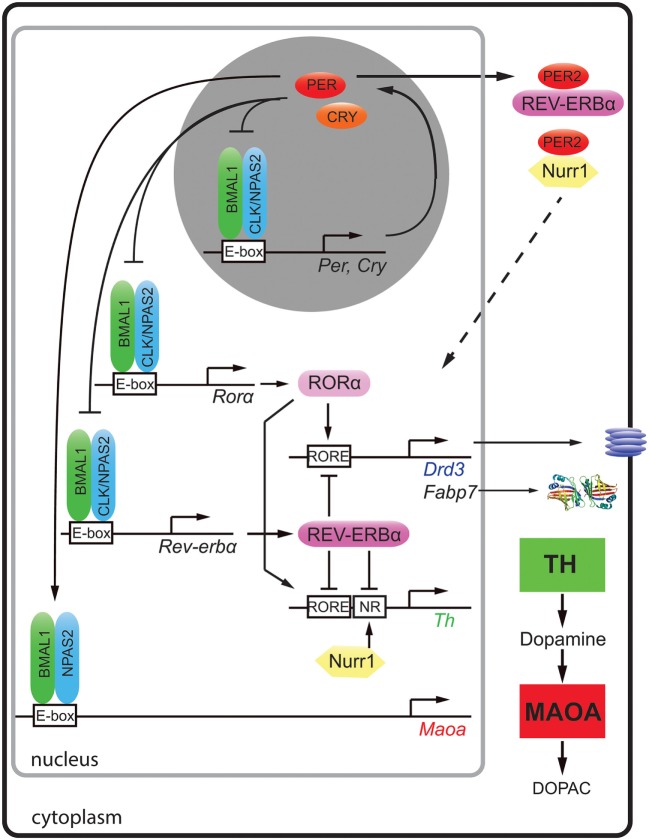
Figure 1.
Molecular regulation of clock and clock-controlled genes of the monoaminergic system and neurogenesis. The clock proteins BMAL1 (green), CLOCK (blue), and NPAS2 (blue) bind to E-box elements present in the promoters of clock genes (Per, Cry, Rorα, and Rev-erbα) and the clock-controlled gene for monoamine oxidase A (Maoa). PER (red) and cryptochrome (CRY, orange) proteins inhibit the action of BMAL1/CLOCK and BMAL1/NPAS2 heterodimers, respectively. The nuclear receptors [retinoic orphan receptor α (RORα, rose)] and REV-ERBα (purple) both bind to RORE elements of dopamine receptor 3 (Drd3), fatty acid binding protein 7 (Fabp7), and tyrosine hydroxylase (Th) in a competitive manner and activate or inhibit their expression, respectively. The nuclear receptor Nurr1 (yellow) regulates Th via its NR promoter element. Via protein–protein interactions, PER2 can modulate the actions of REV-ERBα and Nurr1 (hatched arrow). This regulation results in temporally regulated expression of the dopamine synthesizing (TH, green square) and degrading enzymes (MAOA, red square) leading to fluctuating levels of dopamine in the striatum.