Abstract
Background
In most patients with rheumatoid arthritis (RA), Disease Activity Score 28-joint count C reactive protein (DAS28-CRP) is lower than DAS28 erythrocyte sedimentation rate (DAS28-ESR), suggesting that use of the DAS28-ESR cut-off to assess high disease activity (HDA) with DAS28-CRP may underestimate the number of patients with HDA. We determined the DAS28-CRP value corresponding to the validated DAS28-ESR cut-off for HDA.
Methods
Baseline data were pooled from 2 clinical studies evaluating etanercept (ETN) plus methotrexate (MTX) or MTX in early RA; DAS28-CRP and DAS28-ESR were obtained, allowing the determination of the DAS28-CRP HDA value best corresponding to the DAS28-ESR cut-off of >5.1.
Results
At baseline, as expected, fewer patients had HDA by DAS28-CRP than DAS28-ESR; DAS28-CRP>5.1 and DAS28-ESR>5.1 had only modest agreement (κ coefficients 0.45–0.54). Mean DAS28-CRP and DAS28-ESR were 5.7 and 6.2, respectively, in the ETN+MTX group (n=571), and 6.0 and 6.5 in the MTX group (n=262). A DAS28-CRP cut-off of 4.6 corresponded to a DAS28-ESR cut-off of 5.1.
Conclusions
We have shown that a DAS28-CRP of 4.6 corresponds to 5.1 for DAS28-ESR. Since this is substantially lower than the DAS28-ESR cut-off of 5.1, using 5.1 as the cut-off for DAS28-CRP underestimates disease activity in RA.
Trial registration number
Keywords: Rheumatoid Arthritis, Anti-TNF, DAS28
Key messages.
What is already known about this subject?
The Disease Activity Score 28-joint count (DAS28) values calculated using C reactive protein (CRP) are lower than DAS28 values calculated using erythrocyte sedimentation rate (ESR).
What does this study add?
We determined that the definition of high disease activity (HDA) is >4.6 when using DAS28-CRP, since this is comparable to the validated DAS28-ESR HDA cut-off of >5.1.
How might this impact on clinical practice?
Use of DAS28-ESR cut-offs for DAS28-CRP for HDA underestimates the number of patients with HDA, and patients who should be eligible for advanced therapies may be excluded if eligibility is calculated according to DAS28-CRP.
Use of this new definition of HDA, as well as the DAS28-CRP cut-offs for low disease activity and remission we identified previously, will enable more accurate measurement of disease activity when the DAS28-CRP is used.
Introduction
Clinical trials in rheumatoid arthritis (RA) show that the Disease Activity Score 28-joint count (DAS28) calculated using C reactive protein (CRP) is lower than DAS28 calculated using erythrocyte sedimentation rate (ESR).1–7 This suggests that using DAS28-ESR cut-offs for DAS28-CRP for high disease activity (HDA) underestimates the number of patients with HDA. Nevertheless, these values are often used interchangeably in clinical trials, and by payers and health organisations when evaluating patients for advanced therapies. Consequently, patients who should be eligible for advanced therapies by guidelines used in many countries, for example, the National Institute for Health and Care Excellence (NICE) guidelines in the UK,8 may be excluded if eligibility is calculated by DAS28-CRP.
The American College of Rheumatology (ACR) recommendations on the use of disease activity measures for RA do not distinguish between DAS28-CRP and DAS28-ESR, implying that both measurements use the cut-offs for remission and low disease activity (LDA) of <2.6 and ≤3.2, respectively.9 The 2015 ACR treatment guideline for RA provides cut-offs for DAS28-ESR but does not mention DAS28-CRP.10 The European League Against Rheumatism (EULAR) and Asia Pacific League of Associations for Rheumatology (APLAR) recommendations for managing RA refer to remission and LDA calculated using DAS28 but do not specify whether ESR or CRP should be used.11 12 Consequently, clinicians may assume that values of DAS28-CRP and DAS28-ESR are interchangeable.
Previously, we demonstrated that DAS28-CRP cut-off values equivalent to DAS28-ESR for remission and LDA were <2.4 and ≤2.9, respectively, rather than <2.6 and ≤3.2.13 For this report, we analysed baseline data from two clinical trials with etanercept (ETN) to determine the DAS28-CRP cut-off value corresponding to the validated DAS28-ESR cut-off value for HDA in RA.
Methods
We evaluated baseline data from COMET and PRIZE (ClinicalTrials.gov identifiers: NCT00195494, NCT00913458), randomised clinical trials which enrolled patients with early moderate-to-severe RA who were naïve to methotrexate (MTX) and biological therapy.14 15 Our previous report on the definition of LDA and remission for DAS28-CRP included additional studies. However, the present analysis evaluated the cut-off for HDA and thus required a substantial number of patients with moderate RA; only COMET and PRIZE included sufficient numbers of patients. The current analysis did not assess other measures of RA, such as the simplified disease activity index or the clinical disease activity index, because the confusion about measuring HDA generally relates to use of the DAS28-ESR cut-off when using DAS28-CRP.
COMET was a global study with sites in Asia, Australia, Europe and Latin America; patients were randomised to receive ETN 50 mg+MTX once weekly (QW) or MTX QW.14 Patients had disease duration ≥3 months and ≤2 years, DAS28-ESR≥3.2, and either ESR≥28 mm/hour or CRP≥20 mg/L. PRIZE was conducted in Europe and Asia; all patients initially received ETN 50 mg+MTX QW.15 Patients had DAS28-ESR>3.2 and symptom onset within the previous 12 months.
Statistical methods
Baseline data from the COMET and PRIZE studies were evaluated separately and also pooled. The method of analysis was described in detail previously.13 Briefly, descriptive statistics for DAS28-CRP (calculated using traditional CRP in the COMET study and high-sensitivity (hs)-CRP in the PRIZE study) and DAS28-ESR were determined for each treatment group. DAS28-CRP and DAS28-ESR were compared according to the DAS28-ESR cut-off value for HDA (>5.1) using sensitivity, specificity, κ coefficients and proportion of discordance. Based on the DAS28-ESR cut-off value, the corresponding DAS28-CRP value was determined for each study and treatment group using cumulative distribution plots, receiver operator curves and maximum concordance. These DAS28-CRP values were averaged for each study and treatment, and then averaged overall. This produced a new value for the DAS28-CRP HDA cut-off that corresponded best to the validated DAS28-ESR HDA cut-off.
Results
The COMET trial included 265 patients who received ETN 50 mg+MTX QW and 263 who received MTX QW; the trial results have been reported in detail elsewhere.14 The study participants had early RA with a mean (SE) disease duration of 9.0 (0.3) months and active disease: mean (SD) swollen joint count (SJC) 17.3 (10.2) out of 68 joints, tender joint count (TJC) 25.0 (14.5) out of 71 joints, ESR 48.5 (24.0) mm/hour and CRP 36.7 (36.1) mg/L.14 The percentage of patients with moderate disease activity (MDA, defined as DAS28-ESR≥3.2 to ≤5.1) in the ETN+MTX and MTX groups was 21/265 (7.9%) and 20/263 (7.6%), respectively.
The PRIZE study included 306 patients who received ETN 50 mg+MTX QW. The mean (SD) age was 49.9 (13.7) years; disease duration was 6.5 (2.9) months, DAS28-ESR was 6.0 (1.1), SJC was 11.1 (5.9) out of 28 joints, TJC was 14.1 (7.1) out of 28 joints, ESR was 34.8 (23.2) mm/hour and hs-CRP was 15.2 (22.4) mg/L. The percentage of patients with MDA defined by DAS28-ESR was 64/306 (20.9%).
As expected, our analysis demonstrated that at baseline, fewer patients met the HDA cut-off of >5.1 by DAS28-CRP than by DAS28-ESR (table 1). In the pooled ETN+MTX group, 394/571 (69.0%) patients had DAS-CRP>5.1, and 486/571 (85.1%) had DAS28-ESR>5.1. In the MTX group, 204/262 (77.9%) patients had DAS-CRP>5.1, and 243/263 (92.4%) had DAS28-ESR>5.1. There was only modest agreement between DAS28-CRP>5.1 and DAS28-ESR>5.1 (κ coefficients ranging from 0.45 to 0.54), with high sensitivity but low specificity.
Table 1.
Statistical measures comparing DAS28-CRP>5.1 and DAS28-ESR>5.1 at baseline
| DAS28-CRP>5.1 | DAS28-ESR>5.1 | Proportion of discordance between parameters | Sensitivity (%) | Specificity (%) | κ coefficient | |
|---|---|---|---|---|---|---|
| COMET | ||||||
| ETN+MTX | 208/265 (78.5) | 244/265 (92.1) | 36/265 (13.6) | 100.0 | 36.8 | 0.48 |
| MTX | 204/262 (77.9) | 243/263 (92.4) | 38/262 (14.5) | 100.0 | 34.5 | 0.45 |
| PRIZE | ||||||
| ETN+MTX | 186/306 (60.8) | 242/306 (79.1) | 62/306 (20.3) | 98.4 | 50.8 | 0.54 |
| Pooled | ||||||
| ETN+MTX | 394/571 (69.0) | 486/571 (85.1) | 98/571 (17.2) | 99.2 | 46.3 | 0.53 |
Sensitivity and specificity based on standard DAS28-CRP>5.1.
Values are n/N (%) unless otherwise noted.
DAS28-CRP, Disease Activity Score in 28 joints calculated with C reactive protein; DAS28-ESR, Disease Activity Score in 28 joints calculated with erythrocyte sedimentation rate; ETN, etanercept; MTX, methotrexate.
In the pooled ETN+MTX group, mean (95% CI) DAS28-CRP and DAS28-ESR values were 5.7 (5.6 to 5.8) and 6.2 (6.2 to 6.3), respectively, with a mean (95% CI) difference of 0.57 (0.53 to 0.60). In the MTX group, mean (95% CI) DAS28-CRP and DAS28-ESR values were 6.0 (5.9 to 6.1) and 6.5 (6.4 to 6.6), respectively, with a mean (95% CI) difference of 0.54 (0.50 to 0.58).
We determined that a mean DAS28-CRP cut-off value of 4.6 corresponds to a DAS28-ESR HDA cut-off value of 5.1 (table 2). The mean DAS28-CRP HDA cut-off value was similar between the COMET and PRIZE studies, ranging from 4.62 to 4.68.
Table 2.
DAS28-CRP HDA cut-off estimation that corresponds to DAS28-ESR>5.1
| DAS28-CRP estimation* | Sensitivity+specificity (maximum) | Concordance (maximum) | DAS28-CRP cut-offs†, mean (SD) | |
|---|---|---|---|---|
| COMET | ||||
| ETN+MTX | 4.52 | 4.78 | 4.55 | 4.62 (0.14) |
| MTX | 4.55 | 4.92 | 4.55 | 4.68 (0.21) |
| PRIZE | ||||
| ETN+MTX | 4.51 | 5.01 | 4.34 | 4.62 (0.35) |
| Pooled | ||||
| All | 4.64 (0.03) | |||
*Based on DAS28-CRP and DAS28-ESR cumulative distribution plots.
†Based on mean of all three statistical approaches, presented per treatment group.
DAS28-CRP, Disease Activity Score in 28 joints calculated with C reactive protein; DAS28-ESR, Disease Activity Score in 28 joints calculated with erythrocyte sedimentation rate; ETN, etanercept; HDA, high disease activity; MTX, methotrexate.
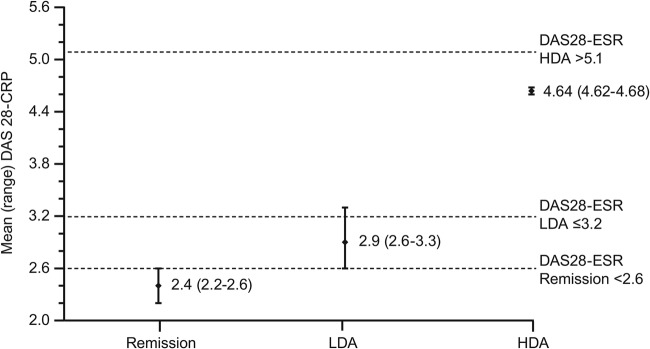
Similar to the HDA cut-off estimation in this analysis, our previous assessment determined that the DAS28-CRP cut-off values for LDA and remission were lower than the corresponding DAS28-ESR cut-off values.13 The DAS28-CRP estimated (range) cut-off values for remission and LDA were 2.4 (2.2–2.6) and 2.9 (2.6–3.3), respectively, figure 1. The DAS28-CRP cut-off values for the COMET study were similar to the overall cut-offs calculated for all studies in the report.13
Figure 1.
DAS28-CRP cut-off values corresponding to the DAS28-ESR cut-off values for remission, LDA and HDA, average of three statistical approaches. Cut-offs for remission and LDA are from Fleischmann et al.13 DAS28-CRP, Disease Activity Score in 28 joints calculated with C reactive protein; DAS28-ESR, Disease Activity Score in 28 joints calculated with erythrocyte sedimentation rate; HDA, high disease activity; LDA, low disease activity.
Discussion
In this analysis, more patients met the HDA cut-off value of >5.1 according to DAS28-ESR than DAS28-CRP, with mean values for DAS28-CRP being lower. These results highlight that DAS28-CRP is not interchangeable with DAS28-ESR. Consequently, use of DAS28-CRP to assess eligibility for advanced therapies by payer guidelines (such as NICE) will exclude a significant number of otherwise eligible and appropriate patients.
Several other published studies also determined that DAS28-CRP and DAS28-ESR are not interchangeable.1–7 Inoue et al1 reported a much lower HDA cut-off value of 4.1 for DAS28-CRP. They also found lower remission and LDA cut-off values (2.3 and 2.7, respectively) than we found previously (2.4 and 2.9), although they were similar to the values we calculated in our subanalysis of the Asian population.13 This suggests that the discrepancy in study results may be due, at least in part, to the difference in study populations. The study by Inoue et al was conducted in Japan, and cut-offs identified in a Japanese population may not be transferrable to the global population.
Castrejón et al7 determined that the best DAS28-CRP cut-off values for their patient population were 2.3, 3.8 and 4.9 for remission, LDA and HDA, respectively. However, that population differed from the current one; Castrejon et al7 16 evaluated an early arthritis cohort at a single site in which a large percentage of the patients had undifferentiated arthritis and only 57% met the ACR classification criteria for RA.
At least one published study and the ACR disease activity measurement guidelines for RA suggest that DAS-CRP and ESR are interchangeable.9 17 The improper use of the DAS28-ESR cut-off value when using DAS28-CRP leads to confusion and may lead to mismanagement of patients. For example, clinical studies such as OPTIMA18 and ADACTA19 used DAS28-ESR cut-off values for DAS28-CRP (which was used in the study), thus initiating medication withdrawal in at least some patients with MDA. This is highly discouraged by the ACR 2015 RA Treatment Guideline and the 2013 EULAR recommendations for the treatment of RA. In addition, Smolen et al20 propose that certain biological therapies have a much greater effect on CRP than ESR, resulting in large differences in the rates of MDA according to DAS28-CRP versus DAS28-ESR.
One strength of this analysis is the variability between the two clinical studies in the assays used to measure CRP. DAS28-CRP was calculated using traditional CRP and hs-CRP in the COMET and PRIZE studies, respectively, thus increasing the generalisability of the results. A limitation is that this analysis only included data from two clinical trials, since only COMET and PRIZE had a sufficient number of patients with moderate RA. The TEMPO trial, which evaluated ETN for the management of patients with established, long-standing moderate-to-severe RA, included few patients with MDA (41 of 677 patients; 6.1%), and so it could not be included in this analysis.21 In TEMPO, the mean difference between baseline DAS28-ESR and DAS28-CRP was 6.8–6.4=0.4. This value is slightly lower than the difference between baseline DAS28-ESR and DAS28-CRP that results when COMET and PRIZE are pooled (0.5–0.6), but it is within the range (0.4–1.2) found in other clinical trials of patients with established moderate-to-severe RA.3 22–24
Since the difference between baseline DAS28-ESR and DAS28-CRP in this analysis is similar to other clinical trials, the new HDA definition may be applicable across various populations. However, this cut-off needs to be validated in longitudinal or registry cohorts. Additionally, it is possible that minor differences may occur in the cut-off value, depending on the laboratory and also on whether traditional CRP or hs-CRP is used. This analysis included only one study that used traditional CRP and one that used hs-CRP; therefore, we were not able to closely evaluate the potential for differences.
In summary, we recommend the use of a new HDA definition of >4.6 when using DAS28-CRP, since this is comparable to the validated DAS28-ESR HDA cut-off of >5.1. The new HDA definition should be used alongside the previously reported thresholds for DAS28-CRP of ≤2.9 for LDA and <2.4 for remission. This will enable more accurate measurement of disease activity when the DAS28-CRP is used. It is essential that clinicians, clinical triallists, payers and regulatory agencies clearly specify which type of DAS28 score is being used and avoid using them interchangeably.
Acknowledgments
The authors wish to thank all patients who participated in the studies, as well as the investigators and medical staff at all of the participating centres. Medical writing support was provided by Jennica Lewis of Engage Scientific Solutions and was funded by Pfizer.
Footnotes
Funding: The clinical trials included in this analysis were funded by Pfizer. Medical writing support was provided by Jennica Lewis of Engage Scientific Solutions and was funded by Pfizer.
Competing interests: RMF was a principal investigator for clinical trials used in this analysis and has received consulting fees unrelated to the development of this article. DvdH has received consulting fees from Pfizer unrelated to the development of this article. AS is an employee of inVentiv Health and was contracted by Pfizer to provide statistical support for the development of this article. LM and EB are employees of Pfizer.
Ethics approval: For the COMET and PRIZE trials, the final protocol, any amendments and informed consent documentation were reviewed and approved by the Institutional Review Board(s) and Independent Ethics Committee(s) at each of the investigational centres participating in the studies.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Inoue E, Yamanaka H, Hara M et al. . Comparison of Disease Activity Score (DAS)28-erythrocyte sedimentation rate and DAS28-C-reactive protein threshold values. Ann Rheum Dis 2007;66:407–9. 10.1136/ard.2006.054205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsui T, Kuga Y, Kaneko A et al. . Disease Activity Score 28 (DAS28) using C-reactive protein underestimates disease activity and overestimates EULAR response criteria compared with DAS28 using erythrocyte sedimentation rate in a large observational cohort of rheumatoid arthritis patients in Japan. Ann Rheum Dis 2007;66:1221–6. 10.1136/ard.2006.063834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wells G, Becker JC, Teng J et al. . Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis 2009;68:954–60. 10.1136/ard.2007.084459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Son KM, Kim SY, Lee SH et al. . Comparison of the disease activity score using the erythrocyte sedimentation rate and C-reactive protein levels in Koreans with rheumatoid arthritis. Int J Rheum Dis 2015. doi: 10.1111/1756-185x.12698. 10.1111/1756-185X.12698 [DOI] [PubMed] [Google Scholar]
- 5.Hensor EMA, Emery P, Bingham SJ et al. . Discrepancies in categorizing rheumatoid arthritis patients by DAS-28(ESR) and DAS-28(CRP): can they be reduced? Rheumatology (Oxford) 2010;49:1521–9. 10.1093/rheumatology/keq117 [DOI] [PubMed] [Google Scholar]
- 6.Tamhane A, Redden DT, McGwin G et al. . Comparison of the disease activity score using erythrocyte sedimentation rate and C-reactive protein in African Americans with rheumatoid arthritis. J Rheumatol 2013;40:1812–22. 10.3899/jrheum.121225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castrejón I, Ortiz A, Toledano E et al. . Estimated cutoff points for the 28-joint Disease Activity Score based on C-reactive protein in a longitudinal register of early arthritis. J Rheumatol 2010;37:1439–43. 10.3899/jrheum.091333 [DOI] [PubMed] [Google Scholar]
- 8.2016. Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for rheumatoid arthritis not previously treated with DMARDs or after conventional DMARDs only have failed. NICE Technology Appraisal Guidance 375. https://www.nice.org.uk/guidance/ta375.
- 9.Anderson J, Caplan L, Yazdany J et al. . Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Care Res 2012;64:640–7. 10.1002/acr.21649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh JA, Saag KG, Bridges SL et al. . 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2016;68:1–26. 10.1002/art.39480 [DOI] [PubMed] [Google Scholar]
- 11.Lau CS, Chia F, Harrison A et al. . APLAR rheumatoid arthritis treatment recommendations. Int J Rheum Dis 2015;18:685–713. 10.1111/1756-185X.12754 [DOI] [PubMed] [Google Scholar]
- 12.Smolen JS, Landewé R, Breedveld FC et al. . EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2014;73:492–509. 10.1136/annrheumdis-2013-204573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleischmann R, van der Heijde D, Koenig AS et al. . How much does Disease Activity Score in 28 joints ESR and CRP calculations underestimate disease activity compared with the Simplified Disease Activity Index? Ann Rheum Dis 2015;74:1132–7. 10.1136/annrheumdis-2013-204920 [DOI] [PubMed] [Google Scholar]
- 14.Emery P, Breedveld FC, Hall S et al. . Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet 2008;372:375–82. 10.1016/S0140-6736(08)61000-4 [DOI] [PubMed] [Google Scholar]
- 15.Emery P, Hammoudeh M, FitzGerald O et al. . Sustained remission with etanercept tapering in early rheumatoid arthritis. N Engl J Med 2014;371:1781–92. 10.1056/NEJMoa1316133 [DOI] [PubMed] [Google Scholar]
- 16.Castrejón I, Ortiz A, García-Vicuña R et al. . Are the C-reactive protein values and erythrocyte sedimentation rate equivalent when estimating the 28-joint disease activity score in rheumatoid arthritis? Clin Exp Rheumatol 2008;26:769–75. [PubMed] [Google Scholar]
- 17.Nielung L, Christensen R, Danneskiold-Samsøe B et al. . Validity and agreement between the 28-joint Disease Activity Score based on C-reactive protein and erythrocyte sedimentation rate in patients with rheumatoid arthritis. Arthritis 2015;2015:401690 10.1155/2015/401690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smolen JS, Emery P, Fleischmann R et al. . Adjustment of therapy in rheumatoid arthritis on the basis of achievement of stable low disease activity with adalimumab plus methotrexate or methotrexate alone: the randomised controlled OPTIMA trial. Lancet 2014;383:321–32. 10.1016/S0140-6736(13)61751-1 [DOI] [PubMed] [Google Scholar]
- 19.Gabay C, Emery P, van Vollenhoven R et al. . Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet 2013;381:1541–50. 10.1016/S0140-6736(13)60250-0 [DOI] [PubMed] [Google Scholar]
- 20.Smolen JS, Collaud Basset S, Boers M et al. . Clinical trials of new drugs for the treatment of rheumatoid arthritis: focus on early disease. Ann Rheum Dis 2016;75:1268–71. 10.1136/annrheumdis-2016-209429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klareskog L, van der Heijde D, de Jager JP et al. . Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet 2004;363:675–81. 10.1016/S0140-6736(04)15640-7 [DOI] [PubMed] [Google Scholar]
- 22.Burmester GR, Blanco R, Charles-Schoeman C et al. . Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 2013;381:451–60. 10.1016/S0140-6736(12)61424-X [DOI] [PubMed] [Google Scholar]
- 23.Keystone EC, Genovese MC, Klareskog L et al. . Golimumab, a human antibody to tumour necrosis factor {alpha} given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD Study. Ann Rheum Dis 2009;68:789–96. 10.1136/ard.2008.099010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Vollenhoven RF, Fleischmann R, Cohen S et al. . Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 2012;367:508–19. 10.1056/NEJMoa1112072 [DOI] [PubMed] [Google Scholar]