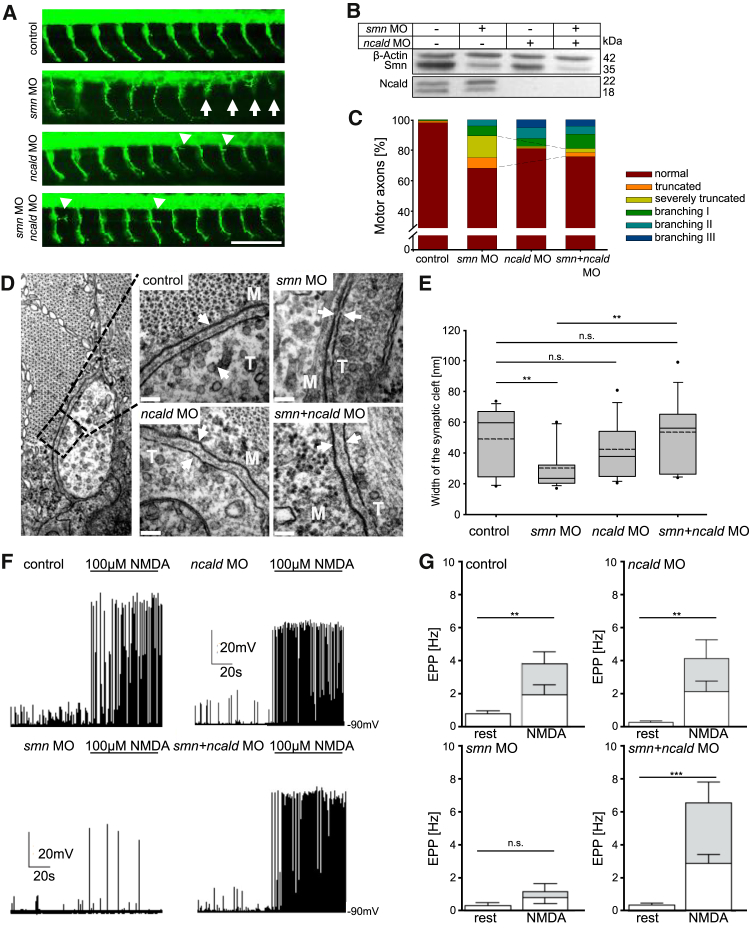
Figure 3.
Ncald Reduction Corrects the Phenotype in Smn-Deficient Zebrafish
(A) First 10 motor axons posterior to the yolk globule of 34 hpf zebrafish embryos injected with respective morpholinos (MO). White arrows mark truncated motor axons. Arrowheads mark extensive branching in ncald or smn+ncald morphants; green shows Znp1 staining, for motor axons. Scale bar represents 100 μm.
(B) Western blot of lysates of zebrafish embryos injected with indicated MO.
(C) Quantification of motor axon phenotype. Dashed lines mark the rescue of the truncation phenotype (∗∗p ≤ 0.01). smn+ncald and ncald morphants showed increased branching. n > 500 motor axons per MO injection.
(D) TEM images of NMJs of 48 hpf zebrafish embryos injected with respective MO. White arrows mark synaptic clefts including basal lamina. M indicates muscle fiber, T indicates nerve terminal. Scale bars represent 100 nm.
(E) Quantification of synaptic cleft width of MO-injected 48 hpf fish (n = 15 per treatment). ∗∗p ≤ 0.01, dashed line indicates mean; straight line indicates median; values covered 24%–75% and dotted outliers at <5% and >95% CI.
(F and G) Whole-cell current clamp recordings EPPs (F) and quantification (G) of mean EPP frequencies in ventral fast muscle cells of control (n = 12), smn (n = 10), ncald (n = 11), and smn+ncald (n = 12) morphants under control conditions or NMDA induction. White bar parts reflect the mEPP frequencies, gray bar parts reflect the frequency of the TTX-sensitive large EPPs. ∗∗p ≤ 0.01; ∗∗∗p ≤ 0.001. Error bars indicate SEM.