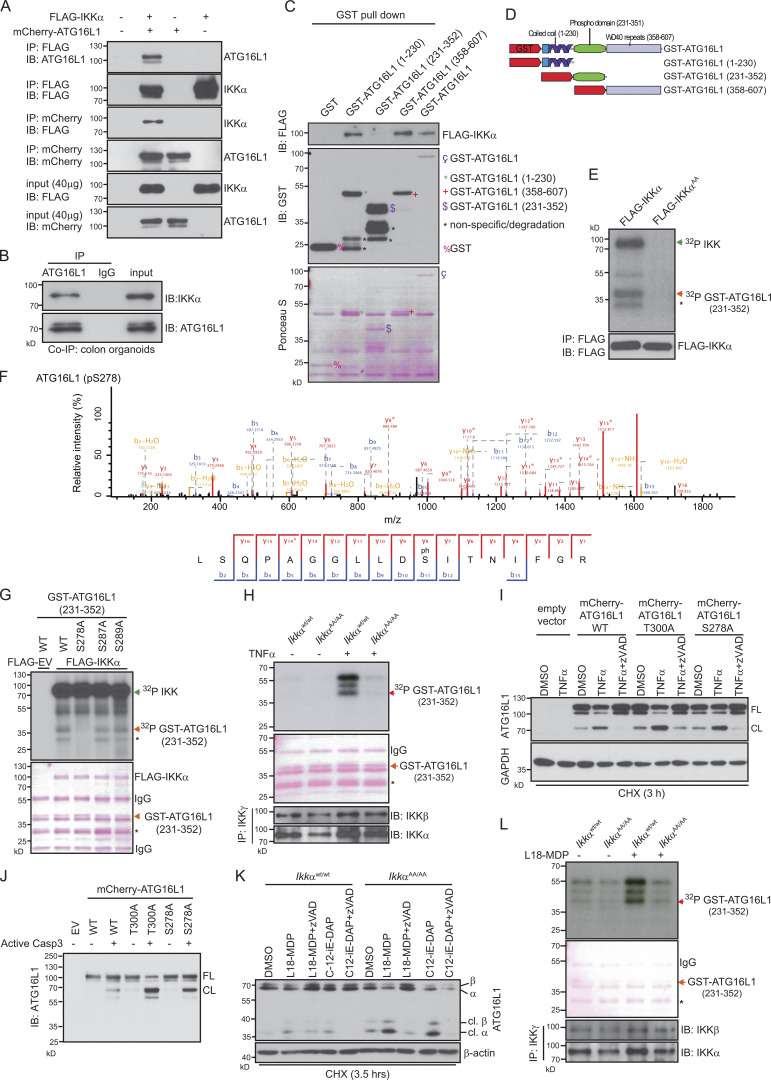
Figure 5.
IKKα phosphorylates ATG16L1. (A) Coimmunoprecipitation (Co-IP) and immunoblot (IB) analysis showing interaction of FLAG-IKKα and mCherry-ATG16L1. HEK293T cells were transfected as indicated, and lysates were subjected to coimmunoprecipitation assays. Immunoblots from 40-µg input were used to examine protein expression levels. (B) Coimmunoprecipitation of endogenous IKKα and ATG16L1 in WT colonic organoids. 600 µg of protein lysates from colon organoids were immunoprecipitated with ATG16L1 or control IgG. The immunoprecipitates and 40 µg of input lysates were analyzed by immunoblot analysis using the indicated antibodies. Data are representative of two experiments. (C) HEK293T cells were transfected with FLAG-IKKα, and whole-cell extracts were subjected to a pull down assay using various forms of GST-ATG16L1 and GST-Sepharose beads. The bead-bound proteins were analyzed by immunoblot analysis using anti-FLAG antibody. Data are representative of two experiments. (D) Schematic representation of the full-length ATG16L1 and different construct domains used for the expression of GST-fusion proteins. (E) HEK293T cells were transfected with FLAG-IKKαWt or FLAG-IKKαAA. After 48 h, the cells were collected and lysed, and whole-cell extracts were subjected to immunoprecipitation using protein A/G plus agarose beads and anti-FLAG antibody. Then, immunoprecipitates were subjected to in vitro kinase assay with GST-ATG16L1(231–352). The FLAG immunoblot shows equal amounts of immunopurified FLAG-IKKαWt or FLAG-IKKαAA. Phosphorylation of GST-ATG16L1(231–352) was confirmed by mass spectrometry. Data are representative of three experiments. (F) Mass spectrometric fragment ion scan of the peptide corresponding to phosphorylated serine 278 in ATG16L1. Data are representative of two experiments. (G) In vitro kinase assay of FLAG-IKKαWt overexpressing HEK293T cells and different point-mutated GST-ATG16L1(231–352) substrates. Phosphorylated GST-ATG16L1(231–352) was visualized by autoradiography. Ponceau S staining shows the equal amounts of GST-ATG16L1 substrates. Data are representative of two experiments. EV, empty vector. (H) Endogenous IKK complex was immunoprecipitated using anti-IKKγ from untreated or 20 ng/ml TNF–treated (15 min) colonic organoids isolated from IkkαWt/Wt and IkkαAA/AA mice and then subjected to in vitro kinase assay using GST-ATG16L1(231–352) as a substrate. Phosphorylated GST-ATG16L1(231–352) was visualized by autoradiography. Ponceau S staining and immunoblotting against IKKα and IKKβ show the equal amount of GST-ATG16L1 and immunoprecipitation efficiency, respectively. Data are representative of two experiments. (I) ATG16L1 proteolytic cleavage was assessed by immunoblot analysis in HeLa cells expressing mCherry-ATG16L1 Wt and mutants. HeLa cells were pretreated for 1 h with DMSO or 10 µM pan-caspase inhibitor (zVADfmk), followed by TNF stimulation (20 ng/ml) in the presence of 10 µg/ml cycloheximide (CHX) for 3 h. (J) Caspase 3–mediated in vitro ATG16L1 cleavage was assessed by immunoblot analysis. mCherry-ATG16L1 Wt and mutants were immunoprecipitated from HEK293T cells. Then, immunoprecipitates were subjected to in vitro cleavage assay using recombinant active caspase 3. Data are representative of two experiments. (I and J) CL, cleaved; FL, full length. (K) ATG16L1 proteolytic cleavage was assessed by immunoblot analysis in colonic organoids. Organoids were pretreated with DMSO or pan-caspase inhibitor (zVADfmk), followed by stimulation with NOD ligands, 20 µg/ml L-18MDP, and 20 µg/ml C12-iE-DAP, in the presence of 10 µg/ml cycloheximide for 3.5 h. Data are representative of two experiments. cl., cleaved. (L) Endogenous IKK complex was immunoprecipitated from untreated or L18-MDP–treated (20 µg/ml for 15 min) colonic organoids and then subjected to in vitro kinase assay as described in H. Data are representative of two experiments. (G, H, and L) Single asterisks indicate nonspecific signal determined by mass spectrometry.