Table 2.
Scope of the organocatalytic isatin insertion into aryl difluoronitromethyl ketones
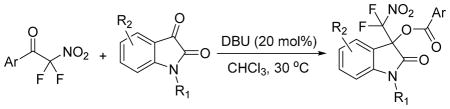
| |||||
|---|---|---|---|---|---|
| Entry | Ar | Isatin R1/R2 | t (h) | Insertion product | Yield (%) |
| 1 | Ph (1) | Bn/H (2) | 24 |
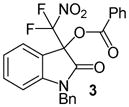
|
99 |
| 2 | 1 | Me/H (4) | 24 |
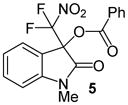
|
99 |
| 3 | 1 | Ph/H (6) | 24 |
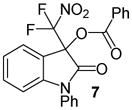
|
99 |
| 4 | 1 | Ph/5-Me (8) | 24 |
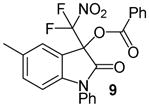
|
91 |
| 5 | 1 | Ph/6-F (10) | 24 |

|
91 |
| 6 | 1 | Ph/5-OMe (12) | 48 |
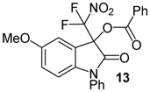
|
91 |
| 7 | 1 | Ph/5-Cl (14) | 48 |
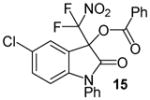
|
97 |
| 8 | 1 | Ph/5,6-F2 (16) | 48 |
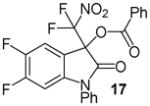
|
81 |
| 9 | 1 | Ph/5-OCF3 (18) | 48 |
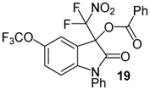
|
85 |
| 10 | 1-Np (20) | Ph/H (6) | 24 |
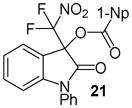
|
96 |
| 11 | 20 | Ph/6-F (10) | 24 |
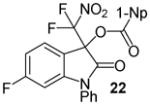
|
91 |
| 12 | 20 | Ph/6-OMe (23) | 48 |
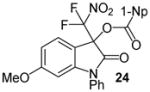
|
99 |
| 13 | 20 | Ph/4-Br (25) | 24 |
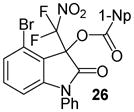
|
99 |
| 14 | 20 | Ph/6-Br (27) | 24 |
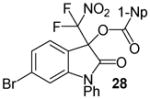
|
99 |
General conditions: A solution of 1 or 20 (0.18 mmol), the isatin (0.15 mmol) and DBU (0.03 mmol) in 0.4 mL of CHCl3 was stirred for 24–48 hours.
